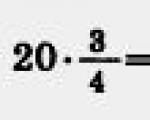Table of isothermal processes. Laws of ideal gases
The main processes in thermodynamics are:
- isochoric, flowing at constant volume;
- isobaric flowing at constant pressure;
- isothermal, occurring at a constant temperature;
- adiabatic, at which there is no heat exchange with the environment;
- polytropic, satisfying the equation pvn= const.
Isochoric, isobaric, isothermal and adiabatic processes are special cases of a polytropic process.
In the study of thermodynamic processes determine:
- process equation in p— v And T— s coordinates;
- relationship between gas state parameters;
- change in internal energy;
- the amount of external work;
- the amount of heat supplied to the process or the amount of heat removed.
Isochoric process


Isochoric process inp, v— , T, s- Andi, s-coordinates (diagrams)
In an isochoric process, the condition v= const.
From the ideal gas equation of state ( pv = RT) follows:
p/T = R/V= const,
i.e., the gas pressure is directly proportional to its absolute temperature:
p2/p1 = T2 /T1.
The expansion work in an isochoric process is zero ( l= 0), since the volume of the working fluid does not change (Δ v= const).
The amount of heat supplied to the working fluid in the process 1-2 at c v
q= c v(T 2 — T 1 ).
T. to. l= 0, then based on the first law of thermodynamics Δ u = q, which means that the change in internal energy can be determined by the formula:
Δ u = c v (T 2 - T 1).
The change in entropy in an isochoric process is determined by the formula:
s2–s1= Δ s = c v ln( p2/p1) = c v ln( T2 /T1).
isobaric process



isobaric process inp, v— , T, s- Andi, s-coordinates (diagrams)
An isobaric process is one that takes place at constant pressure. p= const. From the equation of state for an ideal gas it follows:
v/T = R/p= const
v2/v1 = T 2 /T 1 ,
i.e., in an isobaric process, the volume of a gas is proportional to its absolute temperature.
The work will be:
l = p(v 2 – v 1 ).
T. to. pv 1 = RT 1 And pv 2 = RT 2 , That
l = R(T2-T1).
The amount of heat at cp= const is determined by the formula:
q = cp(T2-T1).
The change in entropy will be:
s2–s1= Δ s = cp ln( T2 /T1).
Isothermal process



Isothermal process inp, v— , T, s- Andi, s-coordinates (diagrams)
In an isothermal process, the temperature of the working fluid remains constant T= const, so:
pv = RT= const
p2/p1 = v1/v2,
i.e., pressure and volume are inversely proportional to each other, so that during isothermal compression the gas pressure increases, and during expansion it decreases.
The work of the process will be equal to:
l = RT ln ( v2 – v1) = RT ln ( p1 – p2).
Since the temperature remains unchanged, the internal energy of an ideal gas in an isothermal process remains constant (Δ u= 0) and all the heat supplied to the working fluid is completely converted into the work of expansion:
q = l.
During isothermal compression, heat is removed from the working fluid in an amount equal to the work expended on compression.
The change in entropy is:
s2–s1= Δ s = R ln( p1/p2) = R ln( v2/v1).
adiabatic process



adiabatic process inp, v— , T, s- Andi, s-coordinates (diagrams)
An adiabatic process is a change in the state of a gas that occurs without heat exchange with the environment. Since d q= 0, then the equation of the first law of thermodynamics for an adiabatic process will have the form:
d u + p d v = 0
Δ u+ l = 0,
hence
Δ u= —l.
In the adiabatic process, the work of expansion is performed only due to the expenditure of the internal energy of the gas, and during compression, which occurs due to the action of external forces, all the work done by them goes to increase the internal energy of the gas.
Let us denote the heat capacity in the adiabatic process through c hell, and condition d q= 0 is expressed as follows:
d q = c hell d T = 0.
This condition says that the heat capacity in the adiabatic process is zero ( c hell = 0).
It is known that
Withp/c v= k
and the equation of the adiabatic process curve (adiabatic) in p, v-diagram looks like:
p.v.k= const.
In this expression k is called adiabatic exponent(also called Poisson's ratio).
Values of the adiabatic exponentkfor some gases:
k air = 1.4
k superheated steam = 1.3
k ICE exhaust = 1.33
k saturated wet steam = 1.135
From the previous formulas follows:
l= — Δ u = c v(T 1 – T 2 );
i 1 – i 2 = cp(T 1 – T 2 ).
The technical work of the adiabatic process ( l tech) is equal to the difference between the enthalpies of the beginning and end of the process ( i 1 – i 2 ).
An adiabatic process that occurs without internal friction in the working fluid is called isentropic. IN T, s in the diagram, it is represented by a vertical line.
Usually, real adiabatic processes proceed in the presence of internal friction in the working fluid, as a result of which heat is always released, which is imparted to the working fluid itself. In this case d s> 0 and the process is called real adiabatic process.
Polytropic process
A process is called polytropic, which is described by the equation:
pvn= const.
Polytropic index n can take any value from -∞ to +∞, but for this process it is a constant value.
From the polytropic process equation and the Claiperon equation, one can obtain an expression that establishes a relationship between p, v And T at any two points on the polytrope:
p2/p1 = (v1/v2)n; T2 /T1 = (v1/v2) n-1 ; T2 /T1 = (p2/p1) (n-1)/n .
The work of gas expansion in a polytropic process is:

In the case of an ideal gas, this formula can be transformed:

The amount of heat supplied or removed in the process is determined using the first law of thermodynamics:
q = (u 2 – u 1) + l.
Because the

is the heat capacity of an ideal gas in a polytropic process.
At c v, k And n= const c n= const, so a polytropic process is sometimes defined as a process with constant heat capacity.
The polytropic process has a generalizing meaning, because it covers the entire set of basic thermodynamic processes.
Graphical representation of a polytrope in p, v coordinates depending on the polytrope index n.

pv 0= const ( n= 0) is the isobar;
pv= const ( n= 1) is the isotherm;
p 0 v= const, p 1/∞ v= const, pv∞= const - isochore;
p.v.k= const ( n = k) is an adiabat.
n > 0 – hyperbolic curves,
n < 0 are parabolas.
Based on the materials of my lecture notes on thermodynamics and the textbook "Fundamentals of Energy". Author G. F. Bystritsky. 2nd ed., rev. and additional - M.: KNORUS, 2011. - 352 p.
What is an isobaric process
Definition
An isobaric (or isobaric) process is a process that occurs in a constant mass of gas at constant pressure.
Let us write the equation for two states of an ideal gas:
\ \
We divide equation (2) by equation (1), we obtain the equation of the isobaric process:
\[\frac(V_2)(V_1)=\frac(T_2)(T_1)\ (3)\]
\[\frac(V)(T)=const\ \left(4\right).\]
Equation (4) is called the Gay-Lussac law.
Internal energy and the amount of heat of the isobaric process
This process occurs with heat input if the volume is increasing or heat removal to decrease the volume. Let's write down the first law of thermodynamics, we will successively obtain expressions for work, internal energy and the amount of heat of the isobaric process:
\[\delta Q=dU+dA=\frac(i)(2)\nu RdT+pdV,\ \left(5\right).\] \[\triangle Q=\int\limits^(T_2)_ (T_1)(dU)+\int\limits^(V_2)_(V_1)(dA)(6)\]
where $\delta Q\ $ is the elementary heat supplied to the system, $dU$ is the change in the internal energy of the gas in the ongoing process, $dA$ is the elementary work done by the gas in the process, i is the number of degrees of freedom of the gas molecule, R is is the universal gas constant, d is the number of moles of gas.
Change in the internal energy of the gas:
\[\triangle U=\frac(i)(2)\nu R((T)_2-T_1)\ (7)\] \
Equation (8) defines the work for an isobaric process. We subtract equation (1) from (2), we obtain one more equation for gas operation in an isobaric process:
\ \[\triangle Q=\frac(i)(2)нR((T)_2-T_1)+\nu R((T)_2-T_1)=c_(\mu p)\nu \triangle T\ ( 10),\]
where $c_(\mu p)$ is the molar heat capacity of the gas in an isobaric process. Equation (10) determines the amount of heat imparted to a gas of mass m in an isobaric process with an increase in temperature by $\triangle T.$
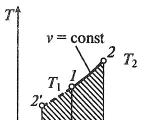
Isoprocesses are often depicted in thermodynamic diagrams. So, a line depicting an isobaric process on such a diagram is called an isobar (Fig. 1).
Example 1
Task: Determine how the pressures $p_1$ and $p_2$ are related on the V(T) diagram in Figure 1c.
Draw the isotherm $T_1$

At points A and B, the temperatures are the same, therefore, the gas obeys the Boyle-Mariotte law:
\ \
Let's convert these volumes to SI: $V_1=2l=2(\cdot 10)^(-3)m^3$, $V_2=4l=4( 10)^(-3)m^3$
Let's do the calculations:
Answer: The work of a gas in an isobaric process is 600 J.
Example 3
Task: Compare the gas work in the ABC process and the gas work in the CDA process Fig. 3.

As a basis for the solution, we take the formula that determines the work of the gas:
From the geometric meaning of the definite integral, it is known that the work is the area of the figure, which is limited by the function of the integrand, the abscissa axis, and isochores at the points $V_1\ and\ V_2$ (axis p(V)). We translate the process graphs into the p(V) axes.
Consider each segment of the graphs of the processes shown in Figure (3).
AB: Isochoric process (p=const), $V\uparrow \left(\ Volume\ grows\right),\ T\uparrow $;
VS: Isochoric process (V =const), $T\uparrow $ (from graph), p$\uparrow $, from the law for isochoric process ($\frac(p)(T)=const$);
CD: (p=const), $V\downarrow ,\ T\downarrow ;$
DA: (V =const), $T\downarrow ,\ p\downarrow .$
Let's depict the graphs of processes in the p(V) axes (Fig. 4):

Gas work $A_(ABC)=S_(ABC)$ ($S_(ABC)$ -- area of rectangle ABFE) (Fig. 3). Work on gas $A_(CDA)=S_(CDA)$ ($S_(CDA)$)$\ -area\ of rectangle\ $EFCD.Obviously $A_(CDA)>A_(ABC).$
, a thermodynamic process is a change in the state of a system, as a result of which at least one of its parameters (temperature, volume or pressure) changes its value. However, if we take into account that all parameters of a thermodynamic system are inextricably linked, then a change in any of them inevitably entails a change in at least one (ideally) or several (in reality) parameters. In the general case, we can say that the thermodynamic process is associated with a violation of the equilibrium of the system, and if the system is in an equilibrium state, then no thermodynamic processes can occur in it.
The equilibrium state of a system is an abstract concept, since it is impossible to isolate anything material from the surrounding world, therefore various thermodynamic processes inevitably occur in any real system. At the same time, in some systems such slow, almost imperceptible changes can take place that the processes associated with them can be conditionally considered to consist of a sequence of equilibrium states of the system. Such processes are called equilibrium or quasi-static.
Another possible scenario of successive changes in the system, after which it returns to its original state, is called circular process or a cycle. The concepts of equilibrium and circular processes underlie many theoretical conclusions and applied methods of thermodynamics.
The study of a thermodynamic process consists in determining the work done in this process, the change in internal energy, the amount of heat, and also in establishing a relationship between individual quantities characterizing the state of a gas.
Of all the possible thermodynamic processes, the isochoric, isobaric, isothermal, adiabatic, and polytropic processes are of greatest interest.
Isochoric process
An isochoric process is a thermodynamic process that occurs at constant volume. Such a process can be performed by heating a gas placed in a closed vessel. The gas heats up as a result of the supply of heat, and its pressure increases.
The change in gas parameters in an isochoric process describes Charles's law: p 1 /T 1 \u003d p 2 /T 2, or in the general case:
p/T = const .
The pressure of a gas on the walls of a vessel is directly proportional to the absolute temperature of the gas.
Since in an isochoric process the change in volume dV is equal to zero, we can conclude that all the heat supplied to the gas is spent on changing the internal energy of the gas (no work is done).
isobaric process
An isobaric process is a thermodynamic process that occurs at constant pressure. Such a process can be carried out by placing the gas in a dense cylinder with a movable piston, which is acted upon by a constant external force during the removal and supply of heat.
When the temperature of the gas changes, the piston moves in one direction or another; while the volume of gas changes in accordance with Gay-Lussac's law:
V/T = const .
This means that in an isobaric process the volume occupied by the gas is directly proportional to the temperature.
It can be concluded that a change in temperature in this process will inevitably lead to a change in the internal energy of the gas, and a change in volume is associated with the performance of work, i.e., in an isobaric process, part of the thermal energy is spent on changing the internal energy of the gas, and the other part is spent on the performance of the gas work to overcome the action of external forces. In this case, the ratio between the heat costs for increasing internal energy and for performing work depends on the heat capacity of the gas.
Isothermal process
An isothermal process is a thermodynamic process that occurs at a constant temperature.
It is very difficult to carry out an isothermal process with gas in practice. After all, it is necessary to comply with the condition that in the process of compression or expansion, the gas has time to exchange temperature with the environment, maintaining its own temperature constant.
The isothermal process is described by the Boyle-Mariotte law: pV \u003d const, i.e. at a constant temperature, the gas pressure is inversely proportional to its volume.
Obviously, in an isothermal process, the internal energy of the gas does not change, since its temperature is constant.
In order to fulfill the condition of constancy of the gas temperature, it is necessary to remove heat from it, equivalent to the work expended on compression:
dq = dA = pdv .
Using the equation of state of the gas, having done a number of transformations and substitutions, we can conclude that the work of the gas in an isothermal process is determined by the expression:
A = RT ln(p 1 /p 2).
adiabatic process
An adiabatic process is a thermodynamic process that proceeds without heat exchange between the working fluid and the environment. Like an isothermal process, it is very difficult to implement an adiabatic process in practice. Such a process can proceed with the working medium placed in a vessel, for example, a cylinder with a piston, surrounded by a high-quality heat-insulating material.
But no matter what high-quality heat insulator we use in this case, some, even if negligible, amount of heat will inevitably be exchanged between the working fluid and the environment.
Therefore, in practice, it is possible to create only an approximate model of the adiabatic process. Nevertheless, many thermodynamic processes carried out in heat engineering proceed so quickly that the working fluid and the medium do not have time to exchange heat, therefore, with a certain degree of error, such processes can be considered as adiabatic.
To derive an equation relating pressure and volume 1 kg gas in an adiabatic process, we write the equation of the first law of thermodynamics:
dq = du + pdv .
Since for an adiabatic process the heat transfer dq is equal to zero, and the change in internal energy is a function of thermal conductivity of temperature: du = c v dT , then we can write:
c v dT + pdv = 0 (3) .
Differentiating the Clapeyron equation pv = RT , we get:
pdv + vdp = RdT .
Let us express dT from here and substitute it into equation (3) . After rearrangement and transformations, we get:
pdvc v /(R + 1) + c v vdp/R = 0.
Taking into account the Mayer equation R = c p – c v, the last expression can be rewritten as:
pdv(c v + c p - c v)/(c p – c v) + c v vdp/(c p – c v) = 0,
c p pdv + c v vdp = 0 (4) .
Dividing the resulting expression by c v and denoting the ratio c p / c v by the letter k , after integrating the equation (4) we get (at k = const):
ln vk + ln p = const or ln pvk = const or pvk = const .
The resulting equation is the equation of an adiabatic process, in which k is the adiabatic exponent.
If we assume that the volumetric heat capacity c v is a constant value, i.e. c v \u003d const, then the work of the adiabatic process can be represented as the formula (given without output):
l \u003d c v (T 1 - T 2) or l \u003d (p 1 v 1 - p 2 v 2) / (k-1).
Polytropic process
Unlike the thermodynamic processes considered above, when any of the gas parameters remained unchanged, the polytropic process is characterized by the possibility of changing any of the main gas parameters. All the above thermodynamic processes are special cases of polytropic processes.
The general equation of the polytropic process has the form pv n = const , where n is the polytropic index - a constant value for this process, which can take values from - ∞ to + ∞ .
It is obvious that by giving certain values to the polytropic index, one or another thermodynamic process can be obtained - isochoric, isobaric, isothermal or adiabatic.
So, if we take n = 0 , we get p = const - an isobaric process, if we take n = 1 , we get an isothermal process described by the dependence pv = const ; for n = k the process is adiabatic, and for n equal to - ∞ or + ∞ . we get an isochoric process.
Topics of the USE codifier: isoprocesses - isothermal, isochoric, isobaric processes.
Throughout this leaflet, we will adhere to the following assumption: the mass and chemical composition of the gas remain unchanged. In other words, we believe that:
That is, there is no gas leakage from the vessel or, conversely, gas inflow into the vessel;
That is, gas particles do not experience any changes (say, there is no dissociation - the decay of molecules into atoms).
These two conditions are satisfied in very many physically interesting situations (for example, in simple models of heat engines) and therefore deserve a separate consideration.
If the mass of a gas and its molar mass are fixed, then the state of the gas is determined by three macroscopic parameters: pressure, volume And temperature. These parameters are related to each other by the equation of state (the Mendeleev-Clapeyron equation).
Thermodynamic process(or simply process) is the change in the state of the gas over time. During the thermodynamic process, the values of macroscopic parameters change - pressure, volume and temperature.
Of particular interest are isoprocesses- thermodynamic processes in which the value of one of the macroscopic parameters remains unchanged. Fixing each of the three parameters in turn, we get three types of isoprocesses.
1. Isothermal process goes at a constant gas temperature: .
2. isobaric process runs at constant gas pressure: .
3. Isochoric process goes at a constant volume of gas: .
Isoprocesses are described by very simple laws of Boyle - Mariotte, Gay-Lussac and Charles. Let's move on to studying them.
Isothermal process
Let an ideal gas perform an isothermal process at a temperature . During the process, only the pressure of the gas and its volume change.
Consider two arbitrary states of the gas: in one of them, the values of the macroscopic parameters are , and in the second, they are . These values are related by the Mendeleev-Clapeyron equation:
As we said from the very beginning, mass and molar mass are assumed to be constant.
Therefore, the right parts of the written equations are equal. Therefore, the left-hand sides are also equal:
(1)
Since the two states of the gas were chosen arbitrarily, we can conclude that during an isothermal process, the product of gas pressure and volume remains constant:
(2)
This statement is called Boyle's Law - Mariotte.
Having written the Boyle-Mariotte law in the form
(3)
one can also formulate it like this: In an isothermal process, the pressure of a gas is inversely proportional to its volume.. If, for example, during isothermal expansion of a gas, its volume increases three times, then the pressure of the gas decreases three times.
How to explain the inverse relationship between pressure and volume from a physical point of view? At a constant temperature, the average kinetic energy of gas molecules remains unchanged, that is, simply speaking, the force of impacts of molecules on the walls of the vessel does not change. With an increase in volume, the concentration of molecules decreases, and, accordingly, the number of molecular impacts per unit time per unit area of the wall decreases - the gas pressure drops. On the contrary, with a decrease in volume, the concentration of molecules increases, their impacts are more frequent, and the pressure of the gas increases.
Isothermal Process Graphs
In general, it is customary to depict graphs of thermodynamic processes in the following coordinate systems:
-diagram: abscissa axis, ordinate axis;
-diagram: abscissa axis, ordinate axis.
The graph of an isothermal process is called isotherm.
An isotherm on a -chart is an inversely proportional plot.
Such a graph is a hyperbola (remember algebra - function graph). Isotherm-hyperbola is shown in fig. 1 .

Rice. 1. Isotherm on -diagram
Each isotherm corresponds to a certain fixed temperature value. It turns out that the higher the temperature, the higher the corresponding isotherm lies on -diagram.
Indeed, let us consider two isothermal processes performed by the same gas (Fig. 2). The first process takes place at a temperature , the second - at a temperature .

Rice. 2. The higher the temperature, the higher the isotherm
We fix some value of the volume . On the first isotherm it corresponds to pressure , on the second - class="tex" alt="p_2 > p_1"> . Но при фиксированном объёме давление тем больше, чем выше температура (молекулы начинают сильнее бить по стенкам). Значит, class="tex" alt="T_2 > T_1"> .!}
In the remaining two coordinate systems, the isotherm looks very simple: it is a straight line perpendicular to the axis ( fig. 3):

Rice. 3. Isotherms on and -diagrams
isobaric process
Recall once again that the isobaric process is a process that takes place at constant pressure. During the isobaric process, only the volume of the gas and its temperature change.
A typical example of an isobaric process: the gas is under a massive piston that can move freely. If the mass of the piston and the cross section of the piston , then the gas pressure is constant and equal to
where is atmospheric pressure.
Let an ideal gas perform an isobaric process at pressure . Consider again two arbitrary states of the gas; this time the values of the macroscopic parameters will be equal to and .
Let us write out the equations of state:
Dividing them by each other, we get:
In principle, this could already be enough, but we will go a little further. Let us rewrite the resulting relation so that only the parameters of the first state appear in one part, and only the parameters of the second state appear in the other part (in other words, we “split the indices” into different parts):
(4)
And now from here - in view of the arbitrariness of the choice of states! - we get Gay-Lussac's law:
(5)
In other words, At constant pressure, the volume of a gas is directly proportional to its temperature.:
(6)
Why does volume increase with temperature? As the temperature rises, the molecules begin to hit harder and raise the piston. At the same time, the concentration of molecules decreases, the impacts become less frequent, so that in the end the pressure remains the same.
Plots of the isobaric process
The graph of the isobaric process is called isobar. On the -diagram, the isobar is a straight line (Fig. 4):

Rice. 4. Isobar on -diagram
The dotted section of the graph means that in the case of a real gas at sufficiently low temperatures, the ideal gas model (and with it the Gay-Lussac law) ceases to work. Indeed, as the temperature decreases, gas particles move more and more slowly, and the forces of intermolecular interaction have an increasingly significant influence on their movement (an analogy: a slow ball is easier to catch than a fast one). Well, at very low temperatures, gases do turn into liquids.
Now let's figure out how the position of the isobar changes with a change in pressure. It turns out that The higher the pressure, the lower the isobar goes. -diagram.
To verify this, consider two isobars with pressures and (Fig. 5):

Rice. 5. The lower the isobar, the greater the pressure
Let us fix some value of temperature . We see that . But at a fixed temperature, the volume is the smaller, the greater the pressure (Boyle's law - Mariotte!).
So class="tex" alt="p_2 > p_1"> .!}
In the remaining two coordinate systems, the isobar is a straight line perpendicular to the axis (Fig. 6):

Rice. 6. Isobars on and -diagrams
Isochoric process
An isochoric process, we recall, is a process that takes place at a constant volume. In an isochoric process, only the pressure of the gas and its temperature change.
An isochoric process is very simple to imagine: it is a process that takes place in a rigid vessel of a fixed volume (or in a cylinder under a piston when the piston is fixed).
Let an ideal gas perform an isochoric process in a vessel of volume . Again, consider two arbitrary gas states with parameters and . We have:
We divide these equations into each other:
As in the derivation of the Gay-Lussac law, we “split” the indices into different parts:
(7)
In view of the arbitrariness of the choice of states, we arrive at Charles law:
(8)
In other words, At a constant volume of a gas, its pressure is directly proportional to its temperature.:
(9)
An increase in the pressure of a gas of a fixed volume when it is heated is a completely obvious thing from a physical point of view. You can easily explain it yourself.
Isochoric Process Plots
The graph of the isochoric process is called isochore. On the -diagram, the isochore is a straight line ( fig. 7):

Rice. 7. Isochore on -diagram
The meaning of the dotted area is the same: the inadequacy of the ideal gas model at low temperatures.

Rice. 8. The lower the isochore, the greater the volume
The proof is similar to the previous one. We fix the temperature and see that . But at a fixed temperature, the pressure is the smaller, the larger the volume (again, the Boyle-Mariotte law). So class="tex" alt="V_2 > V_1"> .!}
In the remaining two coordinate systems, the isochore is a straight line perpendicular to the axis (Fig. 9):

Rice. 9. Isochores on and -diagrams
Boyle's laws - Mariotte, Gay-Lussac and Charles are also called gas laws.
We derived the gas laws from the Mendeleev-Clapeyron equation. But historically it was the opposite: the gas laws were established experimentally, and much earlier. The equation of state appeared subsequently as their generalization.
isobaric process
Plots of isoprocesses in different coordinate systems
isobaric process(Other Greek ισος, isos - “same” + βαρος, baros - “weight”) - the process of changing the state of a thermodynamic system at constant pressure ()
The dependence of gas volume on temperature at constant pressure was experimentally investigated in 1802 by Joseph Louis Gay-Lussac. Gay-Lussac's law: At constant pressure and constant values of the mass of a gas and its molar mass, the ratio of the volume of a gas to its absolute temperature remains constant: V / T = const.
Isochoric process
Isochoric process(from the Greek chorus - occupied place) - the process of changing the state of a thermodynamic system at a constant volume (). For ideal gases, the isochoric process is described by Charles' law: for a given mass of gas at a constant volume, pressure is directly proportional to temperature:
The line depicting an isochoric process in a diagram is called an isochore.
It is also worth pointing out that the energy supplied to the gas is spent on changing the internal energy, that is, Q = 3* ν*R*T/2=3*V*ΔP, where R is the universal gas constant, ν is the number of moles in the gas, T is the temperature in Kelvin , V is the volume of gas, ΔP is the increment of pressure change. and the line depicting the isochoric process in the diagram, in the P(T) axes, should be extended and connected with a dotted line to the origin, as misunderstanding may arise.
Isothermal process
Isothermal process(from the Greek "thermos" - warm, hot) - the process of changing the state of a thermodynamic system at a constant temperature () (). The isothermal process is described by the Boyle - Mariotte law:
At a constant temperature and constant values of the gas mass and its molar mass, the product of the gas volume and its pressure remains constant: PV = const.
Isentropic process
Isentropic process- the process of changing the state of a thermodynamic system at a constant entropy (). For example, a reversible adiabatic process is isentropic: in such a process there is no heat exchange with the environment. An ideal gas in such a process is described by the following equation:
where is the adiabatic exponent, determined by the type of gas.
Wikimedia Foundation. 2010 .
See what "Isoprocesses" are in other dictionaries:
Isoprocesses are thermodynamic processes during which the mass and one more of the physical quantities of the state parameters: pressure, volume or temperature remain unchanged. So, an isobaric process corresponds to a constant pressure, an isochoric volume ... Wikipedia
Molecular kinetic theory (abbreviated as MKT) is a theory that considers the structure of matter from the point of view of three main approximately correct provisions: all bodies consist of particles, the size of which can be neglected: atoms, molecules and ions; particles ... ... Wikipedia
- (abbreviated as MKT) a theory that considers the structure of matter from the point of view of three main approximately correct provisions: all bodies consist of particles whose size can be neglected: atoms, molecules and ions; particles are in continuous ... ... Wikipedia
Books
- Statistical prediction of deformation-strength characteristics of structural materials , G. Pluvinazh , VT Sapunov , This book presents a new method that proposes a common methodology for predicting the characteristics of kinetic processes, common for metallic and polymeric materials. Method… Category: Textbooks for universities Publisher: