As the temperature decreases, the density of the electrolyte How to equalize the density of electrolyte in battery banks? If you don't want to buy a new one
The battery is one of the main elements of the car responsible for starting the engine. The value of the battery is difficult to overestimate, because without it it is impossible to start the engine, which means that the car will not be able to move on its own. That is why the battery requires special attention to itself, excluding the occurrence of unpleasant situations in the form of the impossibility of making a planned trip. At the same time, it should be noted that in order to maintain the performance of this important power source, it is not necessary to take any extra efforts, but it is enough to perform only a small set of preventive measures.
Allowable charge and discharge currents are lower than with other technologies. A short circuit can occur between the two electrodes due to the growth of dendritic lithium. The use of liquid electrolyte is dangerous if it leaks and comes into contact with air or water.
Always use lithium ion batteries with extreme caution, these batteries can be explosive. And as with any battery: Never short circuit a battery, reverse polarity, overload or puncture the case. To avoid problems, these batteries must always be equipped with a protective circuit, a thermal fuse and a safety valve. They must be charged at very precise rates and never discharged below 2.5 volts per cell.
A lead-acid battery is a galvanic cell inside which chemical energy is converted into electrical energy as a result of ongoing reactions. This process is impossible without an electrolyte - an acid solution that ensures the movement of charged particles between the electrodes immersed in it. As a rule, the electrolyte is an aqueous solution of sulfuric acid of a certain density. It is such a parameter as the density of the electrolyte that has a significant impact on the performance of the battery, so it needs to be monitored periodically.
Extend the life of your lithium-ion battery. Do not deep discharge. Do not store batteries for too long without using them. Store the battery at room temperature. Keep the battery at about 40% charge. Do not fully charge the battery before storing it.
Lithium chloride thionyl battery
Do not fully discharge the battery before storing it. Do not stock spare batteries. When buying a battery, check the date of manufacture, its wear, starting from the factory. Do not use while charging. The lithium-thionyl chloride pair contains a lithium metal anode, the lightest of the metals, associated with a liquid cathode consisting of a porous carbon electrode filled with thionyl chloride.
Measuring the density of the electrolyte in the battery
It is not so difficult to measure the density of the electrolyte poured into a lead battery, but there are certain nuances associated with the features of the device and the principle of operation of the battery. Let's list some important points that need to be taken into account:
- It will be possible to carry out the density measurement procedure only in the case of the so-called serviced battery, which provides access to banks (sections) with electrolyte through filler holes closed with lids. It is through these holes (usually their number is six, as well as the number of sections) that the composition is taken to measure the density.
- In the course of its work, the car battery is constantly charging and discharging. The discharge occurs when the starter is cranked, and the charge occurs when the engine is already running from the generator. Depending on the degree of charge, the density of the electrolyte also changes. Values may vary within 0.15-0.16 g/cm 3 . It is important to note that the car alternator is not capable of fully charging the battery. During normal operation on the machine, the battery potential is used only by 80-90%. A full charge can only be provided by an external Charger, which will definitely have to be resorted to before measuring the density of the electrolyte.
- The density of an electrolyte depends on its temperature. Usually measurement is made at a temperature of +25 °C, otherwise corrections are made.
 Suppose all of the above conditions are taken into account, and it is possible to proceed directly to measuring the density. To do this, you need a special device - a densimeter, which consists of a hydrometer, a rubber pear and a glass tube with a tip. The device is introduced into the battery jar through the filler hole, and then the electrolyte is sucked in using a rubber bulb. It continues until the hydrometer floats. Readings are taken after the oscillations of the hydrometer stop and it becomes possible to determine exact value. The readings are taken on a scale, while the gaze should be at the level of the surface of the liquid.
Suppose all of the above conditions are taken into account, and it is possible to proceed directly to measuring the density. To do this, you need a special device - a densimeter, which consists of a hydrometer, a rubber pear and a glass tube with a tip. The device is introduced into the battery jar through the filler hole, and then the electrolyte is sucked in using a rubber bulb. It continues until the hydrometer floats. Readings are taken after the oscillations of the hydrometer stop and it becomes possible to determine exact value. The readings are taken on a scale, while the gaze should be at the level of the surface of the liquid.
They are characterized by extremely low self-discharge, providing a long shelf life or a service life of 10 to 20 years. To facilitate their introduction, batteries can be fitted with different connectors or assembled into batteries, of which there are standardized versions.
lithium manganese dioxide battery
They use large-surface helical electrodes to facilitate the delivery of high currents. Their electrolyte is formulated to provide excellent performance at low temperatures. We won't go into technical details of this system, which are largely explained elsewhere. It suffices to integrate that an ionic electrolyte sodium separator consisting of beta alumina is added to this temperature limitation.
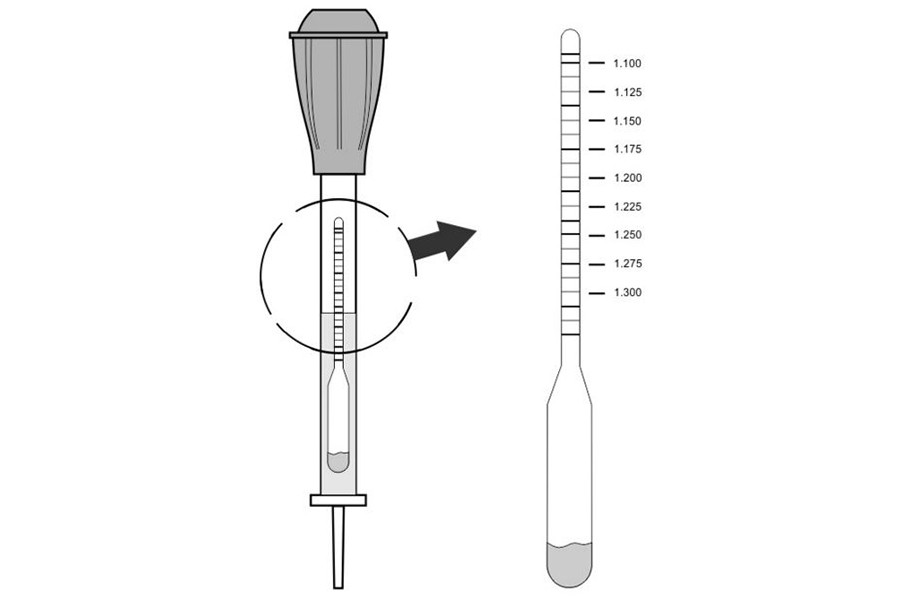 The value obtained should be in the range of 1.25-1.27 g / cm 3 if the car is operated in the middle lane. In the cold climatic zone (the average monthly temperature in January is below -15 ° C), the indicator should be in the range of 1.27-1.29 g / cm 3. It is necessary to check the density of the electrolyte for compliance with these numbers in each of the six cans of the battery. Readings should not differ by more than 0.01 g/cm3, otherwise they will need to be corrected.
The value obtained should be in the range of 1.25-1.27 g / cm 3 if the car is operated in the middle lane. In the cold climatic zone (the average monthly temperature in January is below -15 ° C), the indicator should be in the range of 1.27-1.29 g / cm 3. It is necessary to check the density of the electrolyte for compliance with these numbers in each of the six cans of the battery. Readings should not differ by more than 0.01 g/cm3, otherwise they will need to be corrected.
This mechanically fragile system may be stationary and, if possible, installed outdoors or in a ventilated building. Each module, containing 320 cylindrical memory cells associated with temperature and safety control devices, is filled with sand. A module with a volume of 5.6 m3 weighs more than 3 tons. Fully charged batteries can be discharged to 85% capacity in approximately 6 hours.
The main advantage of sodium-sulfur batteries is the presence of active materials that do not require a transition metal. This is, in my opinion, three more times too expensive to make a real breakthrough. This one is more a new version has a slightly lower voltage, but is safer, less toxic and cheaper. Indeed, the price of lithium-ion batteries and batteries is largely dependent on the materials used on the cathode, which contains cobalt and/or nickel, very expensive metals and makes it more sophisticated to use multiple sources.
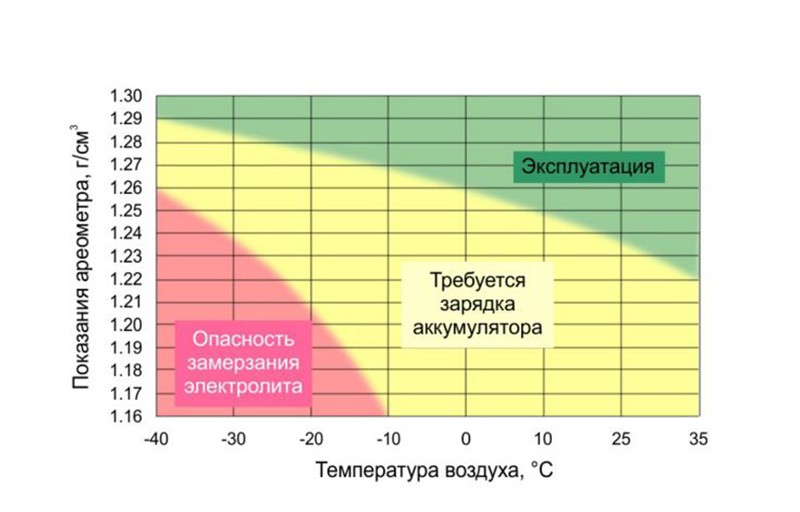
As we have already said, the density of the electrolyte varies with temperature. This means that in winter and summer, the liquid in the same fully functional battery will have a different density. The table below gives an idea of how much the readings will differ.
Another table shows the dependence of the freezing point of the electrolyte on its density. Based on these data, it is possible to establish the optimal electrolyte density for specific climatic conditions. The lower limit of the selected interval should ensure that the electrolyte does not freeze even in the most extreme cold and provide the effort required to turn the starter. At the same time, it is also impossible to overestimate the density, since corrosion processes begin to accelerate on the positive electrodes of the battery, leading to sulfation of the plates.
The phosphate technology lithium battery replaces the standard cathodes with iron phosphate, a cheap material because it does not contain rare metals and is not toxic, unlike cobalt. In addition, this cathode is very stable and does not release oxygen, making it safer.
For industrial development in the electric vehicle, price reduction is necessary. However, research is still ongoing to ensure their lifespan, bring their ability to other lithium-ion methods and, in the long run, improve their behavior at high temperatures: it would seem that the dissolution of iron worsens the cycling of this type of battery.
| Freezing point, °С | Electrolyte density at 25 °С, g/cm 3 | Freezing point, °С | |
|---|---|---|---|
| 1.09 | -7 | 1.22 | -40 |
| 1.10 | -8 | 1.23 | -42 |
| 1.11 | -9 | 1.24 | -50 |
| 1.12 | -10 | 1.25 | -54 |
| 1.13 | -12 | 1.26 | -58 |
| 1.14 | -14 | 1.27 | -68 |
| 1.15 | -16 | 1.28 | -74 |
| 1.16 | -18 | 1.29 | -68 |
| 1.17 | -20 | 1.30 | -66 |
| 1.18 | -22 | 1.31 | -64 |
| 1.19 | -25 | 1.32 | -57 |
| 1.20 | -28 | 1.33 | -54 |
| 1.21 | -34 | 1.40 | -37 |
Reasons for changing the density of the electrolyte
The values recorded as a result of density measurement do not always correspond to the required indicators. Discrepancies can concern both individual cans of the battery, and all together. If the density is too high, then you need to pay attention first of all to the electrolyte level. A low level in most cases is a consequence of electrolysis, leading to the decomposition of the water in the electrolyte into hydrogen and oxygen. This process is expressed in the appearance of bubbles on the surface of the liquid, which usually occurs when the battery is charging. Frequent "boiling" can lead to a decrease in the concentration of water, and this issue is solved by simply adding it. It is worth adding only distilled water to the battery, while controlling the electrolyte level. We will talk more about adjusting the density of the electrolyte below.
This massive energy derived from silicon-based electrochemistry allows it to offer exceptional autonomy, power and safety. Detailed energy / mass 250 W / kg energy / volume 480 W / l nominal voltage per cell 3.40 volts.
This type of battery is particularly suitable for applications that require long battery life, high power, and requiring several hundred charge and discharge cycles, such as security systems or radio communications and other military portable systems.
The first advantage of the lithium-sulfur model is that the anode usually does not consist of lithium, but of a silicon-carbon compound. This connection is significantly more stable because it is smaller when charging. This is an important advantage, because the more the battery anode is changed, the more it interacts with the liquid electrolyte: this process causes the electrolyte to decompose into a gas and a solid, which dries out the battery. In the most extreme cases, the anode is grown to make contact with the cathode, causing a short circuit and discarding the battery.
 If everything is clear with increased density, then with reduced density the situation is somewhat more complicated. In theory, one of the reasons for the decrease in density may be that for some reason the proportion of sulfuric acid in the electrolyte has decreased. However, in practice this is unlikely, since by itself it has a high boiling point, which excludes evaporation even with intense heating, which occurs, for example, when charging battery. A more common reason for a decrease in electrolyte density is the so-called plate sulfation, which consists in the formation of lead sulfate (PbSO4) on the electrodes. In fact, this is a natural process that occurs with each battery discharge. But the fact is that during normal operation, after the battery is discharged, it must be charged (in a car, the battery is constantly recharged from the generator). The charge is accompanied by the reverse transformation of lead sulfate into lead (at the cathode) and lead dioxide (at the anode) - into those active substances that form the basis of the electrodes and are directly involved in the chemical process inside the battery. If the battery is long time in a discharged state, lead sulfate crystallizes, irrevocably losing the ability to participate in chemical reactions. This is a very unpleasant process, as a result of which the battery can no longer be fully charged even when using an external charger due to the fact that not the entire area of \u200b\u200bthe plates is involved in the work. Since the battery is not fully charged, the density of the electrolyte is not restored to its original values. In fact, there is already a conversation about eliminating violations in the normal functioning of the battery.
If everything is clear with increased density, then with reduced density the situation is somewhat more complicated. In theory, one of the reasons for the decrease in density may be that for some reason the proportion of sulfuric acid in the electrolyte has decreased. However, in practice this is unlikely, since by itself it has a high boiling point, which excludes evaporation even with intense heating, which occurs, for example, when charging battery. A more common reason for a decrease in electrolyte density is the so-called plate sulfation, which consists in the formation of lead sulfate (PbSO4) on the electrodes. In fact, this is a natural process that occurs with each battery discharge. But the fact is that during normal operation, after the battery is discharged, it must be charged (in a car, the battery is constantly recharged from the generator). The charge is accompanied by the reverse transformation of lead sulfate into lead (at the cathode) and lead dioxide (at the anode) - into those active substances that form the basis of the electrodes and are directly involved in the chemical process inside the battery. If the battery is long time in a discharged state, lead sulfate crystallizes, irrevocably losing the ability to participate in chemical reactions. This is a very unpleasant process, as a result of which the battery can no longer be fully charged even when using an external charger due to the fact that not the entire area of \u200b\u200bthe plates is involved in the work. Since the battery is not fully charged, the density of the electrolyte is not restored to its original values. In fact, there is already a conversation about eliminating violations in the normal functioning of the battery.
Silver Aluminum Oxide Battery
Sulfur also interacts with the liquid electrolyte, which reduces the performance of batteries until they lose their full power. The silver-zinc pair is used to make primary or secondary batteries. In primary battery cells, the anode consists of zinc and a silver oxide cathode. In secondary battery cells, the anode is made of zinc oxide and a silver cathode. In all cases, the electrolyte is based on potash.
Detailed main applications Military guidance torpedoes, piloting, activation of Ariana rechargeable batteries are used for physical activities. For safety and performance reasons, the battery is only activated at the last moment by electrolyte injection. Silver-zinc batteries are distinguished by their energy density and power.
Partial sulfation of the plates can be eliminated with the help of control and training cycles, which consist in charging and then discharging the battery to a certain level. Most modern chargers have this feature, so it makes sense to use it, especially if the battery has been in a discharged state for some reason. The desulfation procedure is very lengthy and can take up to several days. If it did not bring results, then an extreme measure is to increase the density by adding a corrective electrolyte (density is about 1.40 g/cm3). This method can only be considered as a temporary solution to the problem, because the cause as such is not eliminated.
Silver magnesium chloride battery
Detailed energy weight 3 W/k Energy-volume 103 W/L Service life 3 to 5 years Number of charge cycles 300 to 900 Rated voltage per cell 9 to 1 volts. This technology uses thin sheet boron-silicate fiber between the lead plates of the battery. This thin sheet is impregnated with an electrolyte which comes into contact with the plates.
These batteries have the same qualities as gel batteries and allow more bugs process. Since the electrolyte is impregnated with fiber, it cannot sink even if the battery case breaks, so its transportation is much easier and safer.
How to raise the density of the electrolyte
It is possible to lower or increase the density of the electrolyte in the battery by pumping out a certain amount of it, and adding distilled water or an electrolyte with an increased density (corrective) instead. This procedure is time consuming, since the pumping-refilling cycle can be repeated several times until the required value is reached. After each adjustment, you must put the battery on charge (at least 30 minutes), and then let it stand (0.5-2 hours). These actions are necessary for better mixing of the electrolyte and equalization of the density in the jars.
The gas phase during battery charging transfers oxygen to the negative plates to turn it into water, thus avoiding water loss. This action is more than 99% efficient, making water loss negligible. This phenomenon provides a high degree flexibility in costs and expenses.
This low rate allows for long periods of storage without frequent charging as with standard batteries. In the event of a severe overload, hydrogen emissions are below 4%, which is in line with strict aviation standards and confined spaces.
In the process of raising (or lowering) the density of the electrolyte, one should not forget about controlling its level. It is carried out by a glass tube with two holes along the edges. One edge is immersed in the electrolyte until it hits the safety net. Next, the upper end is closed with a finger, and the tube itself is carefully lifted along with a column of liquid inside. The height of this column indicates the distance from the upper edge of the plates to the surface of the filled electrolyte. It should be 10-15 mm. If the battery has an indicator (tube) or a transparent case with minimum and maximum marks, then it is much easier to control the level.
They have the highest energy density of any battery, but they are not widely used due to cost, shelf life, start-up time, and by-product issues that have limited them to mostly military applications. electric car with aluminum batteries could have ten to fifteen times as many lead-acid batteries with much less overall weight. Once the aluminum anode is consumed by the reaction to atmospheric oxygen on the cathode immersed in the electrolyte for water based, into the hydrated form of alumina, the battery will no longer produce electricity.
Do not forget that all operations with electrolyte must be carried out carefully, using protective gloves and goggles.
Periodicity
Every 15,000 km, check the level and density of the electrolyte.
However, it is possible to mechanically recharge the battery with new aluminum anodes made from recycled hydrated alumina. In fact, the recycling of the generated alumina will be important if aluminum air batteries are widely used. These reactions create a potential difference of 2 volts.
There are still some technical issues that need to be addressed, however, to make air batteries suitable for electric vehicles. Pure aluminum anodes are corroded by the electrolyte, so aluminum is usually alloyed with tin or other proprietary elements. Hydrated alumina, which is created by the reaction of cells, forms a colloidal substance at the anode and reduces the output of electricity. For example, additives have been developed that form alumina as a powder rather than a gel.
Regularly clean the battery from dust and dirt. If the top cover is cracked or bulging, replace the battery.
The electrolyte must be transparent. A brown shade indicates shedding of the active mass of the plates - the battery needs to be changed.
Warnings
During operation, the electrolyte level gradually decreases due to the evaporation of water, which is part of it. To restore the level, add only distilled water to the battery.
In addition, the alloys have been found to form less gel than pure aluminium. These cathodes work well, but they can be expensive. These batteries have been used as backup batteries in several telephone exchanges as a backup power source.
For laptops and mobile phones air-cooled batteries are used and are designed for such use. Detailed energy weight 370 W / k nominal voltage of cells from 35 to 65 volts. Zinc-air batteries and zinc-air fuel cells, electrochemical batteries powered by the oxidation of zinc with atmospheric oxygen. These batteries have a high energy density and are relatively inexpensive to manufacture. They are used in hearing aids and experimental electric vehicles.
When checking the density, be careful: the electrolyte contains sulfuric acid! Drops of electrolyte that have fallen on parts of the car or on open areas of the body, immediately rinse with plenty of water.
Do not smoke or use open flames while charging the battery.
Before charging, remove the battery from the car, otherwise the "boiled" electrolyte may splash out on the body and parts of the car.
They can be important part future zinc economy. The oxidation of zinc in zinc oxide and water is carried back to the system. The water and hydroxyls of the anode are reused at the cathode, so the water supply only serves as a catalyst. Reactions give maximum level 65 volts, but this is reduced to 4-35 volts by reducing the airflow in the cell, this is usually done so that hearing aid batteries reduce the rate of water drying.
The zinc-air fuel cell limitation generally refers to a zinc-air battery in which the zinc fuel is leveled up and the zinc oxide losses are removed without interruption. This is achieved by pushing the electrolyte paste or zinc granules into the anode chamber. Zinc coagulum oxide is pumped into a flexible or waste tank inside the fuel tank, and paste or fresh zinc granules are taken from the fuel tank. Zinc oxide losses are pumped out at fuel replenishment stations and sent to a recycling plant.
Table 1. Electrolyte density correction depending on
temperature
| Electrolyte temperature, °С |
Amendment, g / cm 3 |
|
-40 to -26 |
|
|
-25 to -11 |
|
|
-10 to +4 |
|
|
+5 to +19 |
|
|
+20 to +30 |
|
|
+31 to +45 |
Table 2. Electrolyte density at 25 °С, g/cm 3
|
Climatic region (average monthly air temperature in January, °С) |
Season |
Fully charged battery |
Battery is charged |
|
|
Very cold |
Winter |
|||
|
Cold |
All year round |
|||
|
Moderate |
All year round |
|||
|
warm humid |
All year round |
|||
|
hot dry |
All year round |
|||
Table 3. Approximate norms for adjusting the density of the electrolyte
|
Required electrolyte density in the battery, g / cm 3 |
||||
|
Real electrolyte density, g / cm 3 |
The volume of electrolyte removed from the battery, cm 3 |
|||
PROCEDURE
|
||||||||||||||||||||||||||||