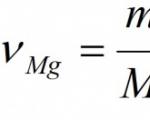Calculations using chemical equations. Calculations using chemical equations Work 6 calculations using equations of chemical reactions
Stoichiometry- quantitative relationships between reacting substances.
If reagents enter into a chemical interaction in strictly defined quantities, and as a result of the reaction substances are formed, the amount of which can be calculated, then such reactions are called stoichiometric.
Laws of stoichiometry:
The coefficients in chemical equations before the formulas of chemical compounds are called stoichiometric.
All calculations using chemical equations are based on the use of stoichiometric coefficients and are associated with finding quantities of a substance (number of moles).
The amount of substance in the reaction equation (number of moles) = the coefficient in front of the corresponding molecule.
N A=6.02×10 23 mol -1.
η - ratio of the actual mass of the product m p to a theoretically possible m t, expressed in fractions of a unit or as a percentage.
If the yield of reaction products is not indicated in the condition, then in the calculations it is taken equal to 100% (quantitative yield).
Calculation scheme using chemical reaction equations:
- Write an equation for a chemical reaction.
- Above the chemical formulas of substances write known and unknown quantities with units of measurement.
- Under the chemical formulas of substances with known and unknowns, write down the corresponding values of these quantities found from the reaction equation.
- Compose and solve a proportion.
Example. Calculate the mass and amount of magnesium oxide formed during complete combustion of 24 g of magnesium.
|
Given: m(Mg) = 24 g Find: ν (MgO) m (MgO) |
Solution: 1. Let's create an equation for a chemical reaction: 2Mg + O 2 = 2MgO. 2. Under the formulas of substances we indicate the amount of substance (number of moles) that corresponds to the stoichiometric coefficients: 2Mg + O2 = 2MgO 2 mole 2 mole 3. Determine the molar mass of magnesium: Relative atomic mass of magnesium Ar (Mg) = 24. Because the molar mass value is equal to the relative atomic or molecular mass, then M (Mg)= 24 g/mol. 4. Using the mass of the substance specified in the condition, we calculate the amount of the substance:
5. Above the chemical formula of magnesium oxide MgO, the mass of which is unknown, we set xmole, above the magnesium formula Mg we write its molar mass: 1 mole xmole 2Mg + O2 = 2MgO 2 mole 2 mole
According to the rules for solving proportions:
Amount of magnesium oxide ν (MgO)= 1 mol. 7. Calculate the molar mass of magnesium oxide: M (Mg)=24 g/mol, M(O)=16 g/mol. M(MgO)= 24 + 16 = 40 g/mol. We calculate the mass of magnesium oxide: m (MgO) = ν (MgO) × M (MgO) = 1 mol × 40 g/mol = 40 g. Answer: ν (MgO) = 1 mol; m (MgO) = 40 g. |
Whatever you study, you
you study for yourself.
Petronius
Lesson objectives:
- introduce students to the basic ways of solving problems using chemical equations:
- find the quantity, mass and volume of reaction products from the quantity, mass or volume of starting substances,
- continue to develop skills in working with the text of a problem, the ability to reasonedly choose a method for solving an educational problem, and the ability to compose equations of chemical reactions.
- develop the ability to analyze, compare, highlight the main thing, draw up an action plan, and draw conclusions.
- cultivate tolerance towards others, independence in decision-making, and the ability to objectively evaluate the results of one’s work.
Forms of work: frontal, individual, pair, group.
Lesson type: combined with the use of ICT
I Organizational moment.
Hello guys. Today, we will learn how to solve problems using equations of chemical reactions. Slide 1 (see presentation).
Lesson objectives Slide 2.
II.Updating knowledge, skills and abilities.
Chemistry is a very interesting and at the same time complex science. In order to know and understand chemistry, you must not only assimilate the material, but also be able to apply the acquired knowledge. You learned what signs indicate the occurrence of chemical reactions, learned how to write equations for chemical reactions. I hope you have a good understanding of these topics and can answer my questions without difficulty.
Which phenomenon is not a sign of chemical transformations:
a) the appearance of sediment; c) change in volume;
b) gas release; d) the appearance of an odor. Slide 3
Please indicate in numbers:
a) equations of compound reactions
b) equations of substitution reactions
c) equations of decomposition reactions Slide 4
In order to learn how to solve problems, it is necessary to create an algorithm of actions, i.e. determine the sequence of actions.
Algorithm for calculations using chemical equations (on each student’s desk)
5. Write down the answer.
Let's start solving problems using an algorithm
Calculating the mass of a substance from the known mass of another substance participating in the reaction
Calculate the mass of oxygen released as a result of decomposition
portions of water weighing 9 g.
Let's find the molar mass of water and oxygen:
M(H 2 O) = 18 g/mol
M(O 2) = 32 g/mol Slide 6
Let's write the equation of the chemical reaction:
2H 2 O = 2H 2 + O 2
Above the formula in the reaction equation we write what we found
the value of the amount of a substance, and under the formulas of substances -
stoichiometric ratios displayed
chemical equation
0.5mol x mol
2H 2 O = 2H 2 + O 2
2mol 1mol
Let's calculate the amount of substance whose mass we want to find.
To do this, we create a proportion
0.5mol = hopmol
2mol 1mol
where x = 0.25 mol Slide 7
Therefore, n(O 2) = 0.25 mol
Find the mass of the substance that needs to be calculated
m(O 2)= n(O 2)*M(O 2)
m(O 2) = 0.25 mol 32 g/mol = 8 g
Let's write down the answer
Answer: m(O 2) = 8 g Slide 8
Calculating the volume of a substance from the known mass of another substance participating in the reaction
Calculate the volume of oxygen (no.) released as a result of the decomposition of a portion of water weighing 9 g.

V(0 2)=?l(n.s.)
M(H 2 O) = 18 g/mol
Vm=22.4l/mol Slide 9
Let's write down the reaction equation. Let's arrange the coefficients
2H 2 O = 2H 2 + O 2
Above the formula in the reaction equation we write the found value of the amount of the substance, and under the formulas of the substances - the stoichiometric ratios displayed by the chemical equation
0.5 mol - x mol
2H 2 O = 2H 2 + O 2 Slide10
2mol - 1mol
Let's calculate the amount of substance whose mass we want to find. To do this, let's create a proportion
![]()
where x = 0.25 mol
Let's find the volume of the substance that needs to be calculated
V(0 2)=n(0 2) Vm
V(O 2) = 0.25 mol 22.4 l/mol = 5.6 l (no.)
Answer: 5.6 l Slide 11
III. Consolidation of the studied material.
Tasks for independent solution:
1. When reducing the oxides Fe 2 O 3 and SnO 2 with coal, 20 g of Fe and Sn were obtained. How many grams of each oxide were taken?
2.In which case is more water formed:
a) when reducing 10 g of copper (I) oxide (Cu 2 O) with hydrogen or
b) when reducing 10 g of copper(II) oxide (CuO) with hydrogen? Slide 12
Let's check the solution to problem 1

M(Fe 2 O 3)=160g/mol
M(Fe)=56g/mol, ![]()
m(Fe 2 O 3)=, m(Fe 2 O 3)= 0.18*160=28.6g
Answer: 28.6g
Slide 13
Let's check the solution to problem 2

M(CuO) = 80 g/mol
4. ![]()
x mol = 0.07 mol,
n(H 2 O)=0.07 mol
m(H 2 O) = 0.07mol*18g/mol=1.26g
Slide 14
CuO + H 2 = Cu + H 2 O
n(CuO) = m/ M(CuO)
n(CuO) = 10g/ 80g/mol = 0.125 mol
0.125mol hops
CuO + H 2 = Cu + H 2 O
1mol 1mol
![]()
x mol = 0.125 mol, n(H 2 O) = 0.125 mol
m (H 2 O) = n * M (H 2 O);
m(H 2 O) = 0.125mol*18g/mol=2.25g
Answer: 2.25g Slide 15
Homework: study the textbook material p. 45-47, solve the problem
What is the mass of calcium oxide and what is the volume of carbon dioxide (n.s.)
can be obtained by decomposing calcium carbonate weighing 250 g?
CaCO 3 = CaO + CO Slide 16.
Literature
1. Gabrielyan O.S. Chemistry course program for grades 8-11 in general education institutions. M. Bustard 2006
2. Gabrielyan O.S. Chemistry. 8th grade. Textbook for general education institutions. Bustard. M. 2005
3. Gorbuntsova S.V. Tests on the main sections of the school course. 8th - 9th grades. VAKO, Moscow, 2006.
4. Gorkovenko M.Yu. Lesson developments in chemistry. To the textbooks of O.S. Gabrielyan, L.S. Guzey, V.V. Sorokin, R.P. Surovtseva and G.E. Rudzitis, F.G. Feldman. 8th grade. VAKO, Moscow, 2004.
5. Gabrielyan O.S. Chemistry. Grade 8: Tests and tests. – M.: Bustard, 2003.
6. Radetsky A.M., Gorshkova V.P. Didactic material on chemistry for grades 8-9: A manual for teachers. – M.: Education, 2000
Application.
Calculations using chemical equations
Algorithm of actions.
In order to solve a calculation problem in chemistry, you can use the following algorithm - take five steps:
1. Write an equation for a chemical reaction.
2. Above the formulas of substances, write known and unknown quantities with the corresponding units of measurement (only for pure substances, without impurities). If, according to the conditions of the problem, substances containing impurities enter into a reaction, then first you need to determine the content of the pure substance.
3. Under the formulas of substances with known and unknowns, write down the corresponding values of these quantities found from the reaction equation.
4. Compose and solve a proportion.
5. Write down the answer.
The relationship between some physical and chemical quantities and their units
Mass (m) : g; kg; mg
Quantity of substances (n): mole; kmol; mmol
Molar mass (M): g/mol; kg/kmol; mg/mmol
Volume (V) : l; m 3 /kmol; ml
Molar volume (Vm) : l/mol; m 3 /kmol; ml/mmol
Number of particles (N): 6 1023 (Avagadro number – N A); 6 1026 ; 6 1020
The lesson is devoted to continuing the study of the topic “Equation of a chemical reaction.” The lesson discusses the simplest calculations using the equation of a chemical reaction, related to the ratio of the amounts of substances participating in the reaction.
Topic: Initial chemical ideas
Lesson: Chemical Reaction Equation
1. The ratio of the amounts of substances participating in the reaction
The coefficients in the reaction equation show not only the number of molecules of each substance, but also the ratio of the amounts of substances participating in the reaction. Thus, according to the reaction equation: 2H2 + O2 = 2H2O - it can be argued that to form a certain amount of water (for example, 2 mol), the same number of moles of the simple substance hydrogen (2 mol) and half as many moles of the simple substance oxygen (1 mol) are needed ). Let us give examples of such calculations.
2. Task 1
TASK 1. Let us determine the amount of oxygen substance formed as a result of the decomposition of 4 moles of water.
ALGORITHM for solving the problem:
1. Write down the reaction equation
2. Make up a proportion by determining the amounts of substances according to the reaction equation and according to the conditions of the problem (designate the unknown amount of substance as x mole).
3. Make an equation (from proportion).
4. Solve the equation, find x.
Rice. 1. Formulation of a brief condition and solution to problem 1
3. Task 2TASK 2. What amount of oxygen is required to completely burn 3 moles of copper?Let's use an algorithm for solving problems using the equation of a chemical reaction.

Rice. 2. Formulation of a brief condition and solution to problem 2.
Carefully study the algorithms and write them down in a notebook, solve the proposed problems yourself
I. Using the algorithm, solve the following problems yourself:
1. Calculate the amount of aluminum oxide substance formed as a result of the interaction of aluminum with a quantity of 0.27 mol of substance with a sufficient amount of oxygen (4Al +3O 2 =2Al2 O3 ).
2. Calculate the amount of sodium oxide substance formed as a result of the interaction of sodium with a 2.3 mol amount of substance with a sufficient amount of oxygen(4Na+O2 =2Na2 O).
Algorithm No. 1
Calculating the amount of a substance from a known amount of the substance involved in a reaction.
Example. Calculate the amount of oxygen released as a result of the decomposition of water with an amount of substance of 6 mol.



II. Using the algorithm, solve the following problems yourself:
1. Calculate the mass of sulfur required to obtain sulfur oxide (IV) with an amount of substance of 4 mol (S + O 2
=SO2).
2. Calculate the mass of lithium required to obtain lithium chloride with an amount of substance of 0.6 mol (2Li+Cl2 = 2LiCl).
Algorithm No. 2
Calculating the mass of a substance from a known amount of another substance involved in a reaction.
Example: Calculate the mass of aluminum required to obtain aluminum oxide with an amount of substance of 8 mol.



III. Using the algorithm, solve the following problems yourself:
1. Calculate the amount of sodium sulfide if sulfur weighing 12.8 g (2Na+S=Na2S) reacts with sodium.
2. Calculate the amount of copper substance formed if copper (II) oxide weighing 64 g reacts with hydrogen (CuO + H2 = Cu + H2 O).
Study the algorithm carefully and write it down in your notebook.
Algorithm No. 3
Calculating the amount of a substance from the known mass of another substance involved in a reaction.
Example. Calculate the amount of copper (I) oxide if copper weighing 19.2 g reacts with oxygen.



Study the algorithm carefully and write it down in your notebook.
IV. Using the algorithm, solve the following problems yourself:
1. Calculate the mass of oxygen required to react with iron weighing 112 g
(3Fe + 4O2 =Fe3 O4).
Algorithm No. 4
Calculating the mass of a substance from the known mass of another substance participating in the reaction
Example. Calculate the mass of oxygen required for the combustion of phosphorus, weighing 0.31 g.



TASKS FOR INDEPENDENT SOLUTION
1. Calculate the amount of aluminum oxide substance formed as a result of the interaction of aluminum with a 0.27 mol amount of substance with a sufficient amount of oxygen (4Al + 3O2 = 2Al2 O3).
2. Calculate the amount of sodium oxide substance formed as a result of the interaction of sodium with a 2.3 mol amount of substance with a sufficient amount of oxygen (4Na + O2 = 2Na2 O).
3. Calculate the mass of sulfur required to obtain sulfur oxide (IV) with an amount of substance of 4 mol (S+O2 =SO2).
4. Calculate the mass of lithium required to obtain lithium chloride with an amount of substance of 0.6 mol (2Li+Cl2 = 2LiCl).
5. Calculate the amount of sodium sulfide if sulfur weighing 12.8 g (2Na+S=Na2S) reacts with sodium.
6. Calculate the amount of copper substance formed if copper (II) oxide weighing 64 g reacts with hydrogen (CuO + H2 = Cu + H2 O).
EXERCISES
Simulator No. 1 - Analysis of the chemical reaction equation
Simulator No. 6 - Stoichiometric calculations
Lesson summary “Calculations using chemical equations”
1. Checking homework
As homework, you were asked to place coefficients in reaction equations.You can see the work done during the break. There will certainly be mistakes.Did everything work out, does anyone have any questions?Let them talk about their home experience.
2. Announcement of the topic and updating of knowledge
The topic of today's lesson is calculations using chemical equations. First, let's remember everything that can be useful to us today. We have already encountered chemical equations in previous laboratory work, in homework, and even earlier in the topic of binary compounds. Let's remember the definition of the equation of a chemical reaction.
(this is a conventional notation of a chemical reaction using chemical formulas and coefficients.)
Amazing.
When producing any compounds, you need to know how much starting material to take to obtain the required mass of the reaction product. To do this, create an equation for the ongoing chemical reaction, and when calculating masses take into account molar masses substances, and when calculating volumes of gases take into account the valuemolar volume gases
Who remembers the value of the molar volume of gases under normal conditions? (22.4 l/mol)
And what are these normal conditions? (pressure 101.3 kPa and temperature 0 o C)
That is, under these conditions, 1 mole of ANY gas occupies a volume of 22.4 liters.
Actually, in order to solve problems, we need to remember several quantities:
Molar mass – M (g/mol)
Amount of substance – n (mol)
Volume – V (l)
It’s better this way: you remember that molar mass is numerically equal to the relative atomic mass or relative molecular mass of a substance. To do this, you need to use the periodic table, where the relative atomic mass is indicated at the bottom of each “cell”. Not forgetting the rounding rules, we use the whole value of this mass in calculations.
Chemistry is a very clear, logical and consistent science, therefore it will be convenient to use the ALGORITHM, which is given in the textbook, to solve problems. This is a universal sequence of actions that is used to solve any problem of this type.
Please open the textbook and let's all get acquainted with the algorithm.
(here we all open our textbooks together, one person, perhaps me, reads the algorithm, the rest follow to understand what they have to do now)
It sounds extensive, but I hope it’s not too confusing. Let's try to figure it out with an example.
Task 1. To produce hydrogen, aluminum is dissolved in sulfuric acid: 2Al + 3H 2 SO 4 → Al 2 (SO 4 ) 3 + 3H 2 (The first point of our algorithm). For the reaction we took 10.8 g of aluminum. Calculate the mass of sulfuric acid consumed.
Given: m(Al) = 10.8 g | Solution: m=10.8 g m - ? 2Al + 3H 2 SO 4 → Al 2 (SO 4 ) 3 + 3H 2 M=27g/mol M=98g/mol Here we can mention that in fact, not 2 aluminum atoms and 3 acid molecules enter into the reaction, but a portion of aluminum atoms and a portion of acid molecules. This portion in chemistry is called the short word “mole”. n=2 mol n=3 mol m = M ∙ n m=54 g m=294 g Calculation by proportion: |
||||
m (H 2 SO 4 ) - ? |
|||||
10.8 g | |||||
54 g | 294 g | ||||
10.8 g ∙ 294 g | |||||
54 g | |||||
X = 58.8 g Answer: m (H 2 SO 4 ) = 58.8 g |
|||||
That's all the solution to the problem. Have questions? Let's talk about the solution one more time:
Made up an equation
Above the substances we signed what we KNOW and what we WANT TO FIND
Under the formulas we wrote down the molar mass, the amount of substance andstoichiometric mass of the substance ( it is better to indicate “mass according to the periodic table”)
Made up a proportion
Solved the proportion
Recorded the answer
Let's solve a similar problem, but with gaseous substances (here we will not use the molar mass of the substance, but what?...molar volume)
Problem 2. 25 grams zinc is dissolved in hydrochloric acid, during the chemical reaction a gas is released - hydrogen. Calculate the volume of hydrogen released.
Given: m(Zn) = 10.8 g | Solution: m=25 g V - ? Zn + 2HCl → ZnCl 2 + H 2 M=65 g/mol V m=22.4 l/mol n=1 mol n=1 mol m=65 g V=22.4 l Calculation by proportion: |
||||
m(HCl) - ? |
|||||
25 g | |||||
65 g | 22.4 l | ||||
25 g ∙ 22.4 l | |||||
65 g | |||||
X = 8.61 l Answer: V(H 2 ) = 8.61 l |
|||||
Let's check how you have mastered the material. Using the same algorithm, solve the problem:
IT IS NOT A FACT THAT YOU WILL BE SUCCESSFUL:
When reacting with using coal oxides Fe2O3 (first option) and SnO2 (second option) each received 20 g of Fe and Sn. How many grams of each oxide were taken?
Please note that we are now calculating the mass of the starting substances, not the reaction products)
(let everyone solve it in a notebook and selectively ask them to show the solution, we will write the equation all together on the board, and everyone will try to solve it themselves)
Fe2O3 + 3C = 2Fe + 3CO m(Fe2O3)= 160*20/2*56= 28.5 g
SnO2+C=Sn+CO2 m(SnO2)= 20*151/119= 25.38 g
Homework: study the textbook material p. 146-150, solve the problem
What is the mass of calcium oxide and what is the volume of carbon dioxide (n.s.)
can be obtained by decomposing calcium carbonate weighing 250 g?SHOULD BE GIVEN TO SCHOOLCHILDRENREADY EQUATIONTO COMPLETE THIS TASK
Methodological lesson development (2 hours)
Calculations using chemical equations
Khapugina Polina Ivanovna,
chemistry teacher GBOU secondary school 277
Kirovsky district of St. Petersburg
Lesson objectives: Teach eighth-graders to make calculations using chemical equations: find the quantity, mass and volume of reaction products from the quantity, volume and mass of the starting substances.
During the classes:
Before moving on to studying a new topic, we need to remember the quantities and formulas you already know. Remember the types of chemical reactions. And you already know how to create chemical equations and equalize them. Let's test your knowledge by doing the following: assessment test.(the contents of the test can be viewed on my personal website in the files and photos folder)
Teacher's explanation:
1. Before learning how to make calculations using chemical equations, you need to once again remember the formulas we already know for finding the amount of a substance, mass and volume of substances; in one step you can test yourself after the work you have done. To do this, let's turn to the following presentation, which will help restore our knowledge in memory. Presentation No. 1.
Write down the formulas you already know in your notebook:
n- amount of substance (mol)
m- mass of substance (g)
M - molar mass (g/mol)
V - gas volume (l)
V m - molar volume = 22.4 l/mol
n = m/M ; m = n.M
n = V/Vm; V = n.V m
2. Now, we must understand that a chemical equation shows not only the qualitative (transformation of substances) side of the process, but also the quantitative side of it. To do this, let's turn to the following Presentations#2(the presentation can be viewed on my personal website in the files and photos folder)
Write in your notebook:
The coefficient in the reaction equation indicates the number of particles, and the number of particles in turn determines the number of moles!
2H₂ + O₂ = 2H₂ ABOUT
Number of particles 2 molecules 1 molecules 2 molecules
Quantity ↓ ↓ ↓
substances,n2 mole 1 mole 2 mole
2 Fe(OH)3 = Fe2 O3 + 3 H2 O
↓ ↓ ↓
n= 2mol 1mol 3mol
3. The next stage that we must examine is the ability to create a proportion using the reaction equation, as well as solve it. To do this, let's turn to the following Presentations 3.(the presentation can be viewed on my personal website in the files and photos folder)
Write in your notebook:
Known by the condition: 2 mol X mol (numerator)
4P+5O₂ = 2P₂ O₅
Known by the equation: 4 mol 5 mol 2 mol (denominator)
Let's compose and solve the proportion:
2 mol X mol
_______ = _______
4 mol 2 mol
X mole = 2 mol. 2 mol= 1 mol
4 mol
X =n(P₂ O₅ )= 1 mol
4. Let's move on to solving problems using equations of chemical reactions. In order to solve a calculation problem in chemistry, you need to use the following algorithm - take five steps. Presentation 4. (the presentation can be viewed on my personal website in the files and photos folder) Textbook p. 101
In your notebook:
Students are given a ready-made solution algorithm to paste into their notebooks.
Algorithm for solving calculation problems using chemical reaction equations:
1. Create an equation for a chemical reaction (i.e., be sure to set the coefficients!)
2. Above the corresponding formulas in the equation, write down quantitative data about substances with units of measurement that are known or can be calculated based on the conditions of the problem, and the desired value X also with units of measurement.
3. Under these formulas, write down the corresponding quantitative quantities specified by the equation itself, also with units of measurement.
4. Compose and solve a proportion.
5. Formalize the answer.
5. Let's solve the problem.
Calculate the mass of water that is formed as a result of the interaction of 0.5 mol of aluminum oxide with sulfuric acid when heated.
Read the problem.
Write down the problem statement. (Given, find.)
In your notebook: (students write down the solution according to the teacher’s explanations according to Presentations 5 ) (the presentation can be viewed on my personal website in the files and photos folder)
Given:
n(Al₂O₃)=0.5 mol
_________________
Find:
Solution:
n=0.5 mol X mol
Al₂ O₃ +3 H₂ SO₄ = Al₂ (SO₄ ) ₃ +3 H₂ O
n= 1 mol 3 mol
M = 102 g/mol 18 g/mol
Calculation of molecular weight:
Мr(Al₂O₃)= 2.27+3.16= 54+48=102
Мr(H₂O)= 2.1+16=18
Compose and solve a proportion.
0.5 mol = X mole
1 mole 3 mole
X mole =n(H₂ O) = 0.5 mol. 3 mol= 1.5 mol
1 mole
Let's find the mass of water.
m(H₂ O) = n(H₂ O) . M(H₂ O)
m(H₂ O) = 1.5 mol.18 g/mol = 27 g
Answer:m(H₂ O)=27 g
6. Solve problems yourself.
Two students are called to the board for assessment.
1. Determine the volume of chlorine (no.) required to obtain 634 g of aluminum chloride according to the equation: 2Al + 3Cl 2 = 2AlCl 3. Answer: 159.6 l
2. Calculate the amount of substance and mass of lithium required for the reaction with oxygen weighing 128 g according to the equation: 4Li + O 2 = 2Li 2 O Answer: 16 mol, 112 g
7. Homework.
Task.
Find the mass of zinc oxide, which is formed when 13 g of zinc reacts with oxygen.