Configuration of macromolecules and stereoisomers. Conformation and flexibility of macromolecules
Classification of polymers according to the chemical structure of the main chain and the macromolecule as a whole. Intermolecular interaction in polymers. Concepts of cohesion energy density and solubility parameter.
Structure of macromolecules includes their chemical structure and length, distribution of lengths and molecular weights, shape and spatial arrangement of units. Based on the chemical structure of the main chain, they are distinguished homochain (with a chain of carbon atoms - carbon chain ) And heterochain polymers, and in terms of the chemical structure of macromolecules as a whole - polymers:
· organic - the chain consists of carbon, oxygen, nitrogen and sulfur atoms;
· organoelement - the chain consists of silicon, phosphorus and other atoms to which carbon atoms or groups are attached, or vice versa;
· inorganic - there are completely no carbon atoms or carbon chain atoms with multiple (double or triple) bonds without side groups.
Most common organic carbon chain polymers, including their various derivatives (halogen-containing, ethers, alcohols, acids, etc.), the name of which is formed by the name of the monomer with the prefix “poly”. Saturated aliphatic carbon-chain polymers include polyethylene, polypropylene, polyvinyl chloride, polytetrafluoroethylene, polytrifluorochloroethylene, polyvinyl alcohol, polyvinyl acetate, polyacrylamide, polyacrylonitrile, polymethyl methacrylate and others. Unsaturated are polybutadiene, polyisoprene and polychloroprene, an example of fatty aromatic polymers is polyethylenephenylene, and aromatic polymers are polyphenylene. Number inorganic homochain polymers are limited - carbon-chain carbin (~C≡C-C≡C~) and cumulene (=C=C=C=), as well as polysulfur (~S-S-S~), polysilane (~SiH 2 -SiH 2 ~), polygerman (~GeH 2 -GeH 2 ~), etc. More common organoelement homochain polymers made from organic chains (carbochain) with organoelement side groups or from inorganic chains with organic radicals: polyvinylalkylsilanes, polyorganosilanes, boron-containing polymers. Organic heterochain Polymers are divided into classes depending on the nature of the functional groups in the main chain. They can be aliphatic or aromatic, depending on the structure of hydrocarbon groups between functional groups (Table 1.1).
Table 1.1.
Heterochain polymers of various classes:
| Functional group | Polymer | |
| Class name | Representatives | |
| Oxygen-containing | ||
| Simple ethereal | Polyethers | Polymethylene oxide (~CH 2 -O~) |
| Polyethylene oxide (~CH 2 -CH 2 -O~) | ||
| Ester | Polyesters | Polyethylene terephthalate ([-CH 2 -CH 2 -O-OC-Ar-CO-O-] n) |
| Polyarylates ([-OC-R-COO-R`-O-] n) | ||
| Polycarbonates ([-O-Ar-CH 2 -Ar-O-CO-O-Ar-CH 2 -Ar-] n) | ||
| Nitrogen containing | ||
| Acetal | Acetals | Cellulose (C 6 H 1 0 O 5) n |
| Amide | Polyamides (-СО-NН-) | Polyhexamethylene adipamide |
| Imide | Polyimides | Polypyromellitimide |
| Urea | Polyurea | Polynonamethylene urea |
| Urethane | Polyurethanes (–HN-CO-O) | ~(CH 2) 4 -O-CO-NH-(CH 2) 2 ~ |
| Sulfur containing | ||
| Thioester | Polysulfides | Polyethylene sulfide (~CH 2 -CH 2 -S~) |
| Sulfonic | Polysulfones | Poly- n,n`-oxydiphenylsulfone |
Inorganic heterochain polymers are polyborazole, polysilicic acid, polyphosphonitrile chloride. Organoelement heterochain polymers include a large group of the most popular compounds from inorganic chains with organic side groups. These include silicon-containing polymers, the chains of which consist of alternating silicon and oxygen atoms ( polyorganosiloxanes ) or nitrogen ( polyorganosilazanes ). Polymers with a third heteroatom in the main chain - a metal - are called polymetalorganosiloxanes (polyaluminum organosiloxanes, polyboron organosiloxanes and polytitanium organosiloxanes). There are also polymers with organoinorganic chains of carbon, silicon, oxygen atoms (polycarbosiloxanes, polycarbosilanes, polycarboranes), which may contain aliphatic or aromatic units. All atoms in the links of the considered polymers are connected chemical covalent bonds . There are also coordination (chelate, intracomplex) heterochain polymers in which the units are connected by donor-acceptor interaction with a metal ion forming coordination link (side valence) and ionic bond (principal valence). Chemical and metallic bonds with a length of 0.1-0.2 nm significantly exceed the energy value of physical bonds and even hydrogen bond (length 0.24-0.32 nm), which occupies an intermediate position between physical and chemical bonds. The polarity of the bonds also depends on the chemical structure and composition of the units, which is quantified by the value of the dipole moment μ O, equal to the product of charge and the distance between charges (Table 1.3), as well as the level of intermolecular interaction in the polymer. Depending on the polarity of the bonds, the polymer can be polar And non-polar . The dipole moment of all organic carbon-chain aliphatic (non-polar) polymers is close to zero. Depending on the structure of macromolecules, dispersion, orientation and inductive bonds may appear between them. Dispersive bonds are caused by the appearance of instantaneous dipoles in atoms when electrons rotate around nuclei. Polar macromolecules are characterized by orientation (dipole-dipole) connections. In the field of dipoles of polar macromolecules, nonpolar macromolecules can also be polarized. Between permanent and induced dipoles, induction communications.
Intermolecular interaction determines the ability of a polymer to dissolve in low molecular weight liquids, behavior at low temperatures, elastic and other properties. Its level is measured solubility parameter – the ratio of the product of the polymer density by the sum of the attraction constants of individual groups of atoms in a composite unit to the molecular weight of the unit. For this they also use cohesion energy density (kJ/mol), which is equivalent to the work of removing interacting macromolecules or groups of atoms from each other at infinitely large distances. At glass transition temperature T s the energy of intermolecular interaction becomes higher than the energy of thermal motion, and the polymer goes into solid vitrified state . Polymers with T With above room temperature is called plastics , and below room temperature and the solubility parameter is 14-19 ( M . J/m 3 ) 1/2 – elastomers (rubbers).
Molecular weight of polymers and methods for its determination. Molecular mass distribution and shape of macromolecules. Classification of polymers according to the number and order of arrangement of constituent units.
Molecular mass(MM) is an important characteristic of the structure of polymers, determining the level of mechanical properties and belonging to a certain group: oligomers (thermosets) - 10 3 -10 4, crystalline thermoplastics - 10 4 -5 . 10 4, amorphous thermoplastics - 5 . 10 4 -2 . 10 5, rubbers – 10 5 -10 6. The lower the MM of polymers, the lower the viscosity of their melts and the easier they are to form. Mechanical properties are determined more by the degree of hardening (oligomers) and crystallinity (polyamides, polyesters) or transition to a glassy state. The highest MM are rubbers that are difficult to mold, but products made from them have high elasticity. Since a large MW does not result in the same degree of polymerization, macromolecules differ in size. Polydispersity (polymolecularity) is one of the basic concepts in the physical chemistry of polymers, and the type molecular weight distribution (MWD) is an important indicator that affects the physical and mechanical properties of polymers no less than MM.
Since MM is an average statistical value, different methods for determining it give different values. WITH sparse numbers methods are based on determining the number of macromolecules in dilute polymer solutions, for example, by measuring their osmotic pressure, and average mass - on determining the mass of macromolecules, for example, by measuring light scattering. Average number MM ( Mn ) is obtained by simply dividing the mass of a polymer sample by the number of macromolecules in it, and mass average MM: M w =M 1 w 1 +M 2 w 2 +…+M i w i , Where w 1 , w 2 , w i – mass fractions of fractions; M 1 , M 2 , M i – mass-average MM fractions. Average viscosity The MM, which approaches the mass average MM, is determined by the viscosity of dilute solutions. The polymer is called monodisperse , if it consists of one fraction with macromolecule sizes very close to each other, and for it the ratio M w/Mn =1.02-1.05. In other cases, the mass average MM is greater than the number average MM, and their ratio ( M w/Mn =2.0-5.0) is a measure of the polydispersity of the polymer. The more M w/Mn , the wider the MMR. On the polymer MWD curve, the value Mn accounts for the maximum, i.e. into the fraction whose share in the polymer composition is the largest, and M w shifted to the right along the x-axis.
The large size of polymer macromolecules determines another feature of their structure. They can be linear or branched (with side branches from the main chain or star-shaped). At close MM values they become isomers . The properties of polymers consisting of linear and branched macromolecules vary greatly. Branching - an undesirable indicator of the structure of macromolecules, reducing their regularity and complicating the crystallization of the polymer. The connection of macromolecules by chemical bonds leads to the formation mesh structures , further changing the properties of polymers. In accordance with such differences in the structure of macromolecules (Fig. 1.1), polymers are called linear , branched And mesh (sewn ).
In the latter case, the concept of “macromolecule” loses its meaning, since the entire cross-linked polymer sample becomes one giant molecule. Therefore, in cross-linked polymers, the average value of the MM of a chain segment between the chemical bonds (network nodes) connecting the macromolecules is determined.
Copolymers contain units of two or more different monomers in the main chain (for example, styrene-butadiene rubber) and have a more complex structure than homopolymers , consisting of units of one monomer. A copolymer with a random combination of monomer units in a macromolecule is called statistical , with their correct alternation - alternating , and with a large extent of sections (blocks) of units of one monomer - block copolymer . If blocks of one of the monomers are attached to the main chain of a macromolecule, composed of units of another monomer, in the form of large side branches, then the copolymer is called vaccinated . The structure of a copolymer is characterized by the chemical composition and length of blocks or graft chains and the number of blocks or grafts in the macromolecule. Units of the same or different monomers can be connected regularly (end of one - beginning of another) or irregularly (the end of one is the end of another, the beginning of another is the beginning of the third link, etc.), and substituents in side groups can have a regular or irregular spatial arrangement. The structure of a macromolecule is also determined by its configuration and conformation.
Configuration of macromolecules and stereoisomers. Conformation and flexibility of macromolecules. Flexible and rigid chain polymers and the shape of their macromolecules.
Macromolecule configuration- this is a certain spatial arrangement of its atoms, which does not change during thermal movement, as a result of which its different types are stable isomers. Cis isomers characterized by the location of different substituents on different sides of the double bond in each repeating unit, and trans isomers - the presence of different substituents on one side of the double bond. An example of such isomers are NA and gutta-percha - natural polyisoprenes identical in chemical structure. Gutta-percha is a plastic with a crystalline structure that melts at 50-70 ° C, and NK is an elastomer in the temperature range from +100 O From to -72 O C, since their macromolecules have different periods of identity . IN cis-polyisoprene (NC) methyl groups oriented in one direction occur through one constituent unit, which is equal to 0.82 nm, and in his trance-isomer (gutta-percha) – after 0.48 nm:
cis- 1,4-polyisoprene (NC)
trance-1.4-polyisoprene
From macromolecules optical polymers with an asymmetric carbon atom, special synthesis methods are used to obtain stereoregular isomers - isotactic (substituents are on one side of the macromolecule plane) and syndiotactic (deputies are on opposite sides):
They differ in properties from atactic polymers with an irregular arrangement of substituents. The mutual repulsion of the substituents leads to their displacement relative to each other in space, and therefore the plane of symmetry bends in the form of a spiral. Structure of spirals is also characteristic of biologically active polymers (for example, the double helix of DNA). The structure of macromolecules of stereoisomers is a carrier of information about the methods of their synthesis, and in proteins, double helices of DNA carry enormous information about their biological heredity.
Macromolecule conformation- this is the spatial arrangement of atoms or groups of atoms, which can change under the influence of thermal motion without destroying the chemical bonds between them. The large length of the macromolecule, with the possibility of rotation of its parts around fixed chemical bonds, determines rotational isomerism , expressed in the appearance of different conformations. The closer the hydrogen atoms are to each other ( cis-position), the greater their repulsion and, accordingly, the potential energy of the macromolecule. The interaction is enhanced by polar substituents, such as chlorine atoms. IN trance-isomers, the potential energy of the macromolecule is less, the arrangement of atoms is more favorable than in cis-isomers. Energy rotation barrier parts of the macromolecule that makes it inhibited , consisting of a series of oscillations, help overcome thermal energy fluctuations . The set of oscillations and movements around simple connections leads to to curvature macromolecules in space, which can go in different directions and change over time. In other words, the macromolecule has flexibility - the ability to change its conformation as a result of thermal movement or the action of external forces. With a large number of atoms, the chain can not only bend, but even curl up very loose macromolecular coil , the size of which can be characterized root mean square distance between its ends and calculate mathematically, knowing the number of constituent links in it. Due to the chain structure of macromolecules, the movement of one atom or group will lead to the movement of others, resulting in a movement similar to the movement of a caterpillar or worm, which is called reptational (Fig. 1.2). A section of a chain that moves as a whole in an elementary act of motion is called chain segment . Thermodynamic flexibility characterizes the ability of a chain to change its conformation under the influence of thermal motion and can be assessed by the rigidity parameter, the length of the thermodynamic segment, or the Flory flexibility parameter. The lower these indicators are, the higher the probability of a macromolecule transitioning from one conformation to another (Table 1.4). Hardness parameter is estimated by the ratio of the root-mean-square distances between the ends of the real and freely jointed chains in dilute polymer solutions. Length of thermodynamic segment A (Kuhn segment) characterizes a sequence of links in which each link behaves independently of the others, and is also related to the root-mean-square distance between the ends of the chain. It is equal to the hydrodynamic length of the macromolecule for extremely rigid chains and the length of the repeating unit for extremely flexible chains. Polymers of the diene series and with ~Si-O~ or ~C-O~ bonds in the main chain are characterized by greater flexibility compared to polymers of the vinyl series, since due to a decrease in exchange interactions between CH 2 -groups have 100 times lower energy of rotary isomers. The nature of the substituents has little effect on the flexibility of macromolecules. Flory flexibility parameter f O shows the content of flexible bonds in a macromolecule and serves as a flexibility criterion by which polymers are divided into flexible chain (f O>0,63; A<10nm) And rigid chain (f O<0,63; A>35nm). The latter are not in the conformation of a macromolecular coil and have an elongated shape of macromolecules - an elastic string (polyalkyl isocyanate, A =100), crankshaft (poly- P-benzamide, A =210) or spirals (biopolymers, A =240).Kinetic flexibility macromolecule reflects the rate of its transition in a force field from one conformation to another and is determined by the value kinetic segment , i.e. that part of the macromolecule that responds to external influences as a whole. Unlike the thermodynamic segment, it is determined by the temperature and speed of external influence. With increasing temperature, the kinetic energy and flexibility of the macromolecule increase and the size of the kinetic segment decreases. Under conditions where the time of action of the force is greater than the time of transition from one conformation to another, kinetic flexibility is high, and the kinetic segment approaches the thermodynamic segment in magnitude. During rapid deformation, the kinetic segment is close to the hydrodynamic length of the macromolecule, and even a thermodynamically flexible chain behaves like a rigid one. The kinetic flexibility of an isolated macromolecule is determined from the viscoelastic properties of highly dilute solutions with their subsequent extrapolation to zero concentration. Macromolecules of a flexible-chain amorphous polymer have ball-shaped both in isolated form and in bulk. Moreover, the structure of the polymer is not similar to the structure of “molecular felt”, in which macromolecules are chaotically entangled, as previously thought. The idea of ordered regions in amorphous polymers was expressed in 1948 by Alfrey.
macromolecule configuration otherwise primary structure(English) - spatial arrangement of atoms in . Determined by the values of bond angles and the lengths of the corresponding bonds.
Description
The configuration of a macromolecule is determined by the relative position of its constituent monomer units, as well as their structure. Currently, the term “structure” or “primary structure” is usually used to describe the configuration of macromolecules.
A distinction is made between short-range (the configuration of the attachment of neighboring units) and long-range configurational order, which characterizes the structure of fairly extended sections of macromolecules. A quantitative measure of tact (order) is the degree of stereoregularity. In addition, tacticity can be described by the number of different types of nearest neighbor pairs (di-, tri-, tetrad), the distribution of which is determined experimentally. A quantitative characteristic of the configuration of statistical network macromolecules, for example, is the cross-linking density, i.e., the average section of the chain between the network nodes.
The configuration of macromolecules is determined by X-ray diffraction analysis, birefringence, etc. As a rule, each method is most “sensitive” to any configuration characteristic; Thus, NMR in many cases makes it possible to quantitatively characterize the short-range configuration order in
· organic polymers(the composition includes organogenic elements - C, N, O, P, S). They are divided into homochain (the main chain contains only carbon atoms) and heterochain (the main chain includes other atoms). This class of polymers includes biopolymers.
· organoelement polymers(in the main chain, along with carbon atoms, there are atoms of Si, Al, Ti, Ge, B).
· inorganic polymers ( the main chain does not contain carbon atoms, such as silicones).
1. List the types of polymer nomenclature.
2. How is nomenclature based on the names of monomers formed?
3. Give examples of polymer names using nomenclature based on the chemical structure of the polymer chain.
4. Name the types of classification of polymers. Give examples.
5. What types of copolymers are there?
6. How is the chemical classification of polymers carried out?
Problems for independent solution*
2. Classification and structural formulas of main polymers
2.1 Classification of polymers
Questions 2501 – 2502, 2403 – 2406, 2307
2.2. Structural formulas of the main polymers
Questions 3501, 3402, 3303 – 3309
*Here and in the following, the tasks are given from the “Collection of test tasks for thematic and final control in the discipline “Chemistry and Physics of Polymers”, M., MITHT, 2009.
Section No. 3. Main characteristics of macromolecules
Macromolecules are characterized by 4 main parameters:
1. Molecular weight (MM), molecular mass distribution (MWD);
2. Macromolecule configuration;
3. Conformation of the macromolecule;
4. Topology (linear, branched).
· MM allows you to determine the length and size of macromolecules;
· Configuration determines the chemical structure of macromolecules;
· Conformation determines the shape of macromolecules.
3.1. Molecular weight (MM), molecular weight distribution (MWD)
The main differences between the concept of MM for the IUD and NMS:
MW is a measure of molecular length for linear polymers and can be expressed in terms of the MW of low molecular weight constituent repeating units:
https://pandia.ru/text/78/135/images/image040_18.gif" width="12" height="2 src=">m0 – molecular weight of the compound repeating unit;
Pn – degree of polymerization
Most synthetic polymers are not individual compounds, but consist of a mixture of molecules of different sizes but the same composition.
This leads to:
· for polymers, the effective molecular weight is an average value due to polydispersity - the spread of macromolecules in molecular weight;
· for most polymers, the end groups differ from the composition of the links in the polymer chain;
· certain side branches may exist in macromolecules, this also distinguishes macromolecules from each other;
· most biopolymers are individual compounds (each specific polymer is unique in composition, structure and molecular weight).
Reasons for polydispersity:
1. due to the statistical nature of the polymer production process: during the synthesis process, macromolecules of various lengths are obtained;
2. due to processes of partial destruction of macromolecules, for example, during the operation of the material;
3. due to the difference in the end groups of the polymer molecule;
4. due to the fact that some polymers have branches in different places and different chemical structures.
3.1.1. Methods for averaging molecular masses
1) Averaging over the number of molecules
Number average MM:
Мw=∑(Ni Mi2)/∑(NiMi) (3.1.1.2)
The mass of the fraction of a given molecular weight is taken into account.
Mw is determined using chromatography, ultracentrifugation, and light scattering methods.
Kn=Mw/Mn (3.1.1.3)
For monodisperse (biological) polymers Kn=1.
With a narrow distribution Kn=1.01÷1.05.
In industry, polymers with Kn=3÷10 are most often produced.
3) Average viscosity mm:
Mŋ=((∑NiMi)1+α/∑(NiMi))1/α, 0<α<1 (3.1.1.4)
![]()
3.1.2. Molecular mass distribution (MWD)
The most complete characteristic of the molecular weights of polymers is the molecular weight distribution function.
Nitrogen, boron, and aluminum can be elements of macromolecular chains in other components of the polymer structure, or be included as heteroatoms in the main chain.

4.3. Carbon
It has a high tendency to form strong covalent bonds, both between its own atoms and with other atoms.
https://pandia.ru/text/78/135/images/image064_12.gif" width="102" height="92"> - two-dimensional carbon-carbon structure of graphene, graphite and soot
It is also possible to obtain a linear chain of carbon atoms:
https://pandia.ru/text/78/135/images/image066_10.gif" width="238" height="14 src=">
When heated, it turns into graphite.
Much greater opportunities for constructing linear macromolecules from carbon atoms open up when 1 or 2 valences of carbon are saturated with other atoms or groups.
 - polyethylene
- polyethylene
 - polypropylene
- polypropylene
![]() - polytetrafluoroethylene
- polytetrafluoroethylene
Also, the main chain may contain various groups containing heteroatoms:
https://pandia.ru/text/78/135/images/image071_11.gif" width="93" height="43 src="> - ester group
https://pandia.ru/text/78/135/images/image073_9.gif" width="105" height="45 src="> - carbamide (urea) group
https://pandia.ru/text/78/135/images/image076_9.gif" width="185 height=84" height="84">
But they are not very chemically stable and during oxidation, silicon binds with oxygen, forming very strong silicon-oxygen bonds.
In nature, silicon occurs in the form of quartz:

This is a rigid three-dimensional structure that does not exhibit the “polymeric” properties of linear macromolecules. Linear macromolecules are obtained by replacing two valencies of each silicon atom with organic radicals (CH3-, C2H5-, etc.). In this case, silicon-organic polymers appear.
Silicon-containing polymers can be synthesized:
 - polysiloxanes
- polysiloxanes
Atoms of Al, B, Ti, Zn and some others can be built into the chain.
4.5. Phosphorus
Phosphorus atoms can form polymers, but the main chain must also include other atoms (most often oxygen):
 - polyphosphates
- polyphosphates
 - polyphosphoric acid
- polyphosphoric acid
Orthophosphoric acid residues are found in natural polymers (nucleic acids, DNA and RNA):

Thus, divalent or polyvalent atoms (C, O, P, N, S, Si, Al, B and some others) can be in the form of elements of the main chain of macromolecules or located in side fragments; monovalent atoms (H, F, Cl, J, Br and some others) can only be arranged as substituents.
Polymer chemistry is built on the basis of these elements.
4.6. Types of polymers
Polymers are obtained either synthetically, or extracted from living organisms (biopolymers), or by processing already isolated natural polymers.
Some synthetically created polymers exist in nature. Polymers are obtained from monomers - low-molecular substances or as a result of transformations of finished polymers (synthetic or natural) - polymer-analogous transformations.

1,4-cis-polybutadiene does not exist in nature; it is obtained synthetically from butadiene.

1,4-cis-polyisoprene exists in nature (natural rubber), but is synthesized in nature from glucose and other substances (but not from isoprene, as in industry)

This polyester can be obtained by condensation of poly-β-hydroxybutyrate, and at the same time it is also synthesized by a number of bacteria.
Syntheses of biopolymers will not be considered in this course.
Many natural polymers are very difficult to produce synthetically. They are produced in living organisms as a result of complex biochemical reactions.
The most important natural polymers:
Examples include reactions polyesterification:
HO-R-COOH + HO-R-COOH > HO-R-COO-R-COOH + H2O, etc.
polyamidation:
H2N-R-NH2 + ClOC-R"-COCl > H2N-R-NHCO-R"-COCl + HCl, etc.
Moreover, unlike polymerization, the elemental composition of polycondensation products in this case does not coincide with the composition of monomer compounds, since each chemical act of polycondensation is accompanied by the release of a molecule of a low molecular weight product.
The above general scheme of polycondensation also corresponds to some types of processes that are not accompanied by the release of low molecular weight products. These include, for example, the synthesis of polyurethanes from glycols and diisocyanates:
HO-R-OH + O=C=N-R"-N=C=O > HO-R-O-CO-NH-R"-N=C=O, etc.
Such polycondensation processes are often called polyaddition. According to the kinetic laws, polyaddition reactions are very similar to polycondensation reactions. In both types of polycondensation processes, the growth of macromolecules is carried out through the interaction of functional groups of monomer molecules or the same groups located at the ends of already formed chains of different molecular weights. The intermediate polymer products obtained as a result of these reactions are quite stable and can be isolated in free form. However, they contain reactive groups at the ends and are therefore capable of further condensation reactions, both with each other and with the corresponding monomer molecules. It follows that theoretically, polycondensation can be considered complete only when all terminal functional groups react, resulting in the formation of one giant cyclic macromolecule. In practice, however, this is never achieved.
Questions for independent study:
1. Which elements of the Periodic Table are capable of forming polymer chains?
2. Give examples of polymers produced synthetically.
3. Give examples of natural polymers.
4. What monomers can participate in the polymerization reaction?
1.3. Configuration of macromolecules
The concept of configuration includes a certain spatial arrangement of atoms of macromolecules, which does not change during thermal movement. A transition from one configuration to another is impossible without breaking chemical bonds.
There are: 1) link configuration, 2) short-range order - the configuration of connecting links, 3) long-range order - the configuration of large sections (for example, blocks and their alternation, or the length and distribution of branches), 5) the configuration of the elongated chain as a whole.
Link configuration. Examples are the cis and trans configurations of diene polymers
1,4-cis-polyisoprene 1,4-trans-polyisoprene (natural rubber) (gutta-percha) Another example would be l,d-isomerism. For example,
for polymers with ~CH2 –CHR~ units, where R is any radical, the formation of two isomers is possible: l – levorotatory, and d – dextrorotatory
Link connection configuration(short range order). Links in the chain can be connected using the “head to tail” or “head to head” type:

is a head-to-tail connection, and a head-to-head connection requires overcoming large activation barriers.
For copolymers, the types of structural isomers increase compared to homopolymers. For example, for copolymers of butadiene and styrene it is possible:
1. sequential alternation of links –A–B–A–B–A–B–,
2. combination of links in the form of dyads and triads–AA–BBV–AA–BBV– ,
3. statistical combination of links–AA–B–AA–BBB–A–B– . Long range configuration order spreads on
tens and hundreds of atoms in the main chain. For example, large sequences of blocks in block copolymers or large sequences of units with the same stereoregularity (for example, polymers with isotactic, atactic and syndiotactic structures).
Isotactic Atactic Syndiotactic
Overall circuit configuration determined by the mutual arrangement of large sequences of links (with long-range order). For example, for branched macromolecules, various types of configurations are shown in Fig. 4.
Rice. 4. Configurations of macromolecules

1.4. Conformation of macromolecules
Conformation is a variable distribution in space of atoms or groups of atoms that form a macromolecule. The transition from one conformation to another can occur due to rotation, rotation or vibration of units around single bonds under the influence of thermal motion or external forces and is not accompanied by the breaking of chemical bonds.
Polymers can take different conformations:
Statistical ball is a folded conformation. Formed when the intensity of internal thermal movement prevails over external influence. Characteristic of linear polymers [PE, PP, PB, PIB and ladder polymers (polyphenylene siloxane).
Helix – formed in polymers due to H-bonds (for example, in protein molecules and nucleic acids).
A globule is a very compact particle in shape, close to spherical. Characteristic of polymers with strong intramolecular interactions (for example, PTFE).
A rod or string is found in alkyl polyisocyanates.
Fold conformation. Characteristic of polymers in a crystalline state (for example, PE).
Crankshaft Conformation is realized in poly-n-benzenamide.
Fig.5. Conformations of macromolecules
1.5. Flexibility of macromolecules
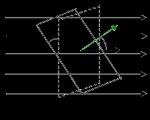
Flexibility is one of the most important characteristics of polymers, determining the highly elastic, relaxation and thermomechanical properties of polymers, as well as the properties of their solutions. Flexibility characterizes the ability of macromolecules to change their shape under the influence of thermal movement of links or external mechanical influences. Flexibility is due to the internal rotation of links or parts of macromolecules relative to each other. Let us consider the phenomenon of internal rotation in molecules using the example of the simplest organic compound - an ethane molecule.
In the ethane molecule (CH3 –CH3), carbon atoms are connected to hydrogen atoms and to each other by covalent (σ-bonds), and the angle between the directions of σ-bonds (bond angle) is 1090 28/. This causes a tetrahedral arrangement of substituents (hydrogen atoms) in space in the ethane molecule. Due to thermal motion in the ethane molecule, one CH3 group rotates relative to the other around the C-C axis. In this case, the spatial arrangement of atoms and the potential energy of the molecule continuously changes. Graphically, various extreme arrangements of atoms in a molecule can be represented in the form of projections of the molecule onto a horizontal plane (Fig. 6). Let us assume that in position a the potential energy of the molecule is equal to U1, and in position b – U2, while U1 ≠ U2, i.e. the positions of the molecule are energetically unequal. Position b, in which the H atoms are located under each other, is energetically unfavorable, since repulsive forces appear between the H atoms, which tend to transfer the atoms to the energetically favorable position a. If we accept
U1 =0, then U2 =max.

Rice. 6. Projection formulas for the extreme locations of H atoms in space in an ethane molecule.
Rice. 7. Dependence of the potential energy of a molecule on the angle of rotation of the methyl group.
When one CH3 group rotates by 600 relative to another, the molecule moves from position a to b and then after 600 again to position a, etc. The change in the potential energy of an ethane molecule depending on the rotation angle φ is shown in Fig. 7. Molecules with less symmetry (for example, a dichloroethane molecule) have a more complex relationship U=f(φ).
Potential (U 0 ) or activation barrier rotation
tion is the energy required for the transition of a molecule from the minimum to the maximum position of potential energy. For ethane, U0 is small (U0 = 11.7 kJ/mol) and at
At normal temperatures, CH3 groups rotate around the C-C bond at high speed (1010 rpm).
If the molecule has an energy reserve less than U0, then there is no rotation and only vibration of the atoms occurs relative to the position of the minimum energy - this is limited or
slow rotation.
In polymers, due to intra- and intermolecular interactions, the dependence U=f(φ) has a complex shape.
If one position of a chain link is characterized by potential energy U1, and the other by U2, then the energy of transition from one position to another is equal to the difference ∆U= U1 – U2. The difference in transition energies ∆U from one equilibrium position of a macromolecule unit to another characterizes thermodynamic flexibility. It determines the ability of the chain to bend under the influence of thermal movement.
Another characteristic of flexibility is the speed of transition of links from one position to another. The rate of conformational transformations depends on the ratio of the value of U0 and the energy of external influences. The larger U0, the slower the links turn and the less flexibility. The flexibility of macromolecules, determined by the value U0, is called kinetic flexible
Factors determining the flexibility of macromolecules
Such factors include: the value of U0, molecular mass of the polymer, the density of the spatial network, the size of substituents and temperature.
Potential rotation barrier (U 0). The value of U0 depends on intra- and intermolecular interactions. Let us consider the factors affecting U0 and chain flexibility in carbon chain polymers.
Carbon chain polymers
In carbon-chain polymers, the least polar are saturated hydrocarbons. Their intra- and intermolecular interactions are small, and the values of U0 and ∆U are small, therefore the polymers have great kinetic and thermodynamic flexibility. Examples: PE, PP, PIB.
U0 values are especially low for polymers in the chain of which there is a double bond next to the single bond.
–CH2 –CH=CH–CH2 – Polybutadiene Introduction into macromolecules of substituents containing
polar groups lead to intra- and intermolecular interactions. In this case, the degree of polarity significantly influences
When introducing polar groups, there are three possible effects on flexibility:
1. Polar groups are closely located and strong interactions are possible between them. The transition of such polymers from one spatial position to another requires overcoming large U0, therefore the chains of such polymers are the least flexible.
2. Polar groups are rarely located in the chain and interactions between them do not appear. The values of U0 and ∆U are small and the polymers have greater kinetic and thermodynamic flexibility.

–СF 2 –СF 2 –
Example: Polychloroprene
3. Polar groups are arranged so that the electric fields cancel each other. In this case, the total dipole moment of the macromolecule is equal to zero. Therefore, the values of U0 and ∆U are low and the polymers have greater kinetic and thermodynamic flexibility.
Example: Polytetrafluoroethylene
Heterochain polymers
In heterochain polymers, rotation is possible around the C–O, C–N, Si–O, and C–C bonds. The U0 values for these bonds are small and the chains have sufficient kinetic flexibility. Examples: polyesters, polyamides, polyurethanes, silicone rubbers.
However, the flexibility of heterochain polymers may be limited by intermolecular interactions due to the formation of H-bonds (for example, cellulose, polyamides). Cellulose is one of the rigid chain polymers. It contains a large number of polar groups (–OH) and therefore cellulose is characterized by intra- and intermolecular interactions, high U0 values and low flexibility.
Molecular weight of polymer. Increasing the molecular weight of the polymer increases chain folding and therefore long macromolecules
have greater kinetic flexibility compared to short macromolecules. As the MW increases, the number of conformations that a macromolecule can take increases and the flexibility of the chains increases.
Spatial grid density. The more chemical bonds between macromolecules, the less flexibility of the chains, i.e. As the density of the spatial grid increases, flexibility decreases. An example is the decrease in chain flexibility with an increase in the number of crosslinks in the resol series< резитол<резит.
Effect of size and number of substituents. An increase in the number of polar and large substituents reduces the mobility of macromolecule units and reduces kinetic flexibility. An example is the decrease in the flexibility of macromolecules of a copolymer of butadiene and styrene with an increase in the content of bulky phenyl substituents in the chain.
If there are two substituents at one carbon atom in the polymer backbone (for example, OCH3 and CH3 in PMMA units), then the macromolecule becomes kinetically rigid.
Temperature. As the temperature increases, the kinetic energy of the macromolecule increases. As long as the kinetic energy value is less than U0, the chains undergo torsional vibrations. When the kinetic energy of the macromolecule becomes equal to or exceeds the value U0, the links begin to rotate. With increasing temperature, the value of U0 changes little, but the speed of rotation of the links increases and the kinetic flexibility increases.
Control questions
1 General information about polymers, concepts, definitions.
2 Define and give examples of organic, non-
organic and organoelement polymers.
2 Classification of homochain polymers, examples.
3 Classification of heterochain polymers, examples.
4 Thermodynamic and kinetic flexibility of macromolecules. What factors influence the flexibility of macromolecules?
5 What is the configuration of macromolecules and what are the possible types of configuration of macromolecules? Examples.
6 What is the conformation of macromolecules and what types of conformations of macromolecules are possible? Examples.
7 What parameters characterize molecular weight, molecular weight distribution and polydispersity of polymers?
8 Molecular characteristics of oligomers.
9 Fractionation of polymers and construction of molecular curves cular mass distribution.
Within a given configuration, a macromolecule has a large number of internal degrees of freedom associated with rotation around the axis of the single bonds of the main chain. As a consequence, the macromolecule is capable of taking various forms ( conformation), i.e. Polymers are characterized by conformational isomerism.
Conformation is the spatial arrangement of atoms and atomic groups, which can be changed without breaking chemical bonds of the main value as a result of thermal movement and (or) external influences.
Below is a schematic diagram of the mechanism for changing the conformation of the isotactic triad of a vinyl polymer as a result of a 180° rotation around the C-C bond. It is obvious that such conformational transitions are not accompanied by a change in the given configuration and breaking of chemical bonds.
Thus, Conformational isomerism of macromolecules is determined by internal rotation around single chemical bonds of the polymer chain structure.
Basic principles of conformational isomerism of macromolecules
Let us consider the basic patterns of internal rotation around chemical bonds using the example of a low-molecular model - 1,2-dichloroethane.

Due to the interaction of side substituents (Hi C1) with a complete rotation around the -C-C bond axis by 360°, a number of different rotary isomers are sequentially realized in the 1,2-dichloroethane molecule, or conformers, with a certain potential energy. Graphically, this can be represented in the form of an energy map - the dependence of the potential energy of the conformer on the rotation angle. For 1,2-dichloroethane, a similar map is shown schematically in Fig. 1.3.

Rice. 1.3. Dependence of potential energy U valence-unbonded atoms of the 1,2-dichloroethane molecule on the rotation angle
Molecules of this type have three stable conformations: one trance- and two gauche conformations (from French. gauche- oblique, skewed), corresponding to the minima of the potential curve. The maxima correspond to unstable eclipsed conformations, in particular the r^is conformer.
In polymers, internal rotation around single bonds has a number of specific features compared to low molecular weight compounds. Let us consider a fragment of a polyvinyl chloride chain in a “head-to-head” configuration.

Unlike 1,2-dichloroethane, in the isolated fragment, instead of two atoms II, the substituents on the carbon atoms are continuations of the polymer chain -CH 2 -. In other words, when rotating around the bond between the gth and (g + 1)th carbon atoms, the (g + 2)th carbon atom with subsequent continuation of the chain plays the role of a substituent (Fig. 1.4).

Rice. 1.4.
The position of the (r + 2)th atom relative to the previous bond is specified by the base of the cone, taking into account the bond angle of 0. However, a rotation of 360° is possible only when an extended continuation of the chain moves in space, which requires enormous thermal energy, which, as a rule, exceeds the dissociation energy of chemical connections. As a result, internal rotation in polymers is inhibited and is implemented within a certain arc of a circle. The size of this arc determines angle of inhibited internal rotation f. The magnitude of the angle of inhibited internal rotation depends on temperature, the nature of the chemical bond, the polarity and volume of substituents, the configurational composition of the polymer, etc.
Thus, to a first approximation, internal rotation in polymer chains is reduced to the rotations of each subsequent bond relative to the previous one. In reality, these events have a pronounced cooperative character, since the rotation of two neighboring bonds relative to each other is largely determined by both similar processes in the near environment and long-range interactions. In this regard, in the case of a polymer, the angle of inhibited internal rotation is an average value. Quantitative estimates of this characteristic will be given below.